This section explains a recommended protocol for glycan profiling experiments targeting formalin-fixed paraffin-embedded tissue (FFPT) sections using lectin microarray. Here, an extremely feasible methodology is described, which provides a straightforward approach to differential glycan analysis using an ultra-sensitive lectin microarray targeting a restricted region on FFPT. Significant advantage of the system is its ultra-sensitivity sufficient to detect glycoproteins derived from only 500 cells comparable to an area of 1.5 mm2 in diameter with 5 μm in thickness). |
| Category | Sugar binding proteins |
| Protocol Name | Tissue glycan analysis using lectin microarray |
Authors
 |
Matsuda, Atsushi
Glycoscience and Glycotechnology Research Group, Biotechnology Research Institute for Drug Discovery, Department of Life Science and Biotechnology, National Institute of Advanced Industrial Science and Technology (AIST)
|
| KeyWords |
|
Reagents
 |
| ● |
0.01 M sodium citrate buffer, pH 6.0 |
| ● |
PBS: 0.01 M phosphate-buffered saline, pH 7.2–7.4 |
| ● |
|
| ● |
|
| ● |
Probing solution (GP Biosciences Ltd., Sapporo, Japan) |
| ● |
FFPT: formalin-fixed paraffin-embedded tissue section |
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
|
Instruments
 |
| ● |
Centrifuge (Tomy Seiko Co., Ltd., Tokyo, Japan) |
| ● |
Sonicator (Tamagawa Seiki, Co., Ltd., Nagano, Japan) |
| ● |
LecChipsTM (GP Biosciences Ltd.) |
| ● |
GlycostationTM Reader (GP Biosciences Ltd.) |
| ● |
Array-Pro Analyzer Ver 4.5 (Media Cybernetics Inc., Bethesda, MD) |
|
| Methods |
|
1. |
Deparaffinization of FFPTs |
| 1) |
Soak the FFPT in Xylene for 10 min (repeat twice). |
Comment 0
|

|
| 2) |
Soak the FFPT in 100% ethanol for 10 min (repeat twice). |
Comment 0
|

|
| 3) |
Soak the FFPT in 95% ethanol for 5 min. |
Comment 0
|

|
| 4) |
Soak the FFPT in 90% ethanol for 5 min. |
Comment 0
|

|
| 5) |
Soak the FFPT in 70% Ethanol for 5 min. |
Comment 0
|

|
| 7) |
Drying the FFPT at room temperature. |
Comment 0
|
|
|
|
2. |
Protein extraction from FFPT |
| 1) |
Scratch for a target area of tissue fragments from FFPT (an area of 1.5 mm in diameter, 5 mm thickness). |
Comment 0
|

|
| 2) |
The scratched tissue fragments are collected into a 1.5 mL tube containing 200 μL of 0.01 M sodium citrate buffer (pH 6.0). |
Comment 0
|

|
| 3) |
Incubate the tube for 1 h at 95˚C for antigen retrieval. |
Comment 0
|

|
| 4) |
Centrifuge the tube at 20,000 × g for 5 min at 4˚C. Remove the supernatant, and add 20 μL of 0.5% NP40-PBS buffer for solubilization. |
Comment 0
|

|
| 5) |
Sonicate the solution for 10 sec (repeat 3 times). |
Comment 0
|

|
| 6) |
Incubate the tissue suspension on ice for 1 h. |
Comment 0
|

|
| 7) |
Centrifuge the tube at 20,000 × g for 5 min at 4˚C (keep the supernatant). |
Comment 0
|
|
|
|
3. |
Lectin microarray analysis |
| 1) |
Transfer the total amount of the sample solution (20 μL) obtained above into PCR tubes containing 10 μg of Cy3-SE, and mix thoroughly. |
Comment 0
|

|
| 2) |
Incubate the tube at room temperature in the dark for 1 h (labeling). |
Comment 0
|

|
| 3) |
Adjust the solution to 200 μL with probing buffer. |
Comment 0
|

|
| 4) |
To block free Cy3, incubate the tube at room temperature in the dark at least 2 h. |
Comment 0
|

|
| 5) |
Adjust the concentration of sample with probing buffer, and then apply to the lectin microarray (60 mL/well). |
Comment 0
|

|
| 6) |
Incubate at 20˚C for overnight in a humid chamber. |
Comment 0
|

|
| 7) |
Scan the microarray with GlycostationTM. |
Comment 0
|

|
| 8) |
All of data were analyzed with the Array Pro analyzer version 4.5. |
Comment 0
|
|
|
| Initial amount | ・ More than 1 mm2, 5 μm in thickness in FFPT section |
| Discussion | The developed protocol provides researchers in extensive fields with a highly practical approach to comparative glycan profiling using limited areas of tissue sections of as small as 1.0 mm2 with 5 μm in thickness. In fact, only one-third of the dissected tissue extract is necessary for the lectin microarray analysis, which corresponds to approximately 500 cells. In its ultra-high sensitivity, the method will also be applicable to glycan profiling targeting even laser micro-dissected FFPTs for more systematic analysis. |
| Figure & Legends |
Figure & Legends

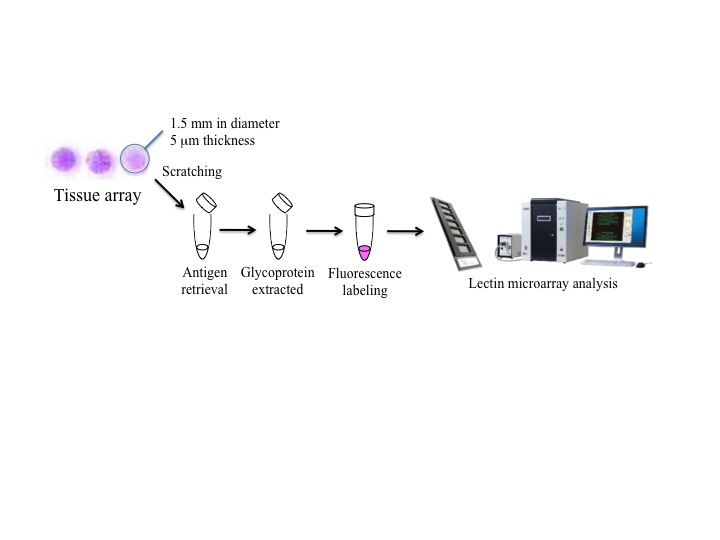
Fig. 1. An all-in-one technology for glycan profiling targeting formalin-embedded tissue sections (FFPT).
A scheme of total procedures using tissue microarray: each one-dot section (1.5 mm diameter and 5 μm thickness, composed of approximately 500 cells) derived from tissue array was subjected to lectin microarray analysis.
This figure was originally published in Biochemical and Biophysical Research Communications. Atsushi M., Atsushi K. et al. "Development of an all-in-one technology for glycan profiling targeting formalin-embedded tissue sections" 2008, 370 (2): 259–263. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-10-30 10:51:49 |
- Matsuda, A., Kuno, A., Ishida, H., Kawamoto, T., Shoda, J., and Hirabayashi, J. (2008) Development of an all-in-one technology for glycan profiling targeting formalin-embedded tissue sections. Biochem. Biophys. Res. Commun. 370, 259–263 [PMID : 18375199]
- Kuno, A., Matsuda, A., Ikehara, Y., Narimatsu, H., and Hirabayashi, J. (2010) Differential glycan profiling by lectin microarray targeting tissue specimens. Methods Enzymol. 478, 165–179 [PMID : 20816479]
- Matsuda, A., Kuno, A., Kawamoto, T., Matsuzaki, H., Irimura, T., Ikehara, Y., Zen, Y., Nakanuma, Y., Yamamoto, M., Ohkohchi, N., Shoda, J., Hirabayashi, J., and Narimatsu, H. (2010) Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology 52, 174–182 [PMID : 20578261]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Matsuda, Atsushi,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Matsuda, Atsushi,
(2014).
Tissue glycan analysis using lectin microarray.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t218.
html source
Matsuda, Atsushi,
(2014).
<b>Tissue glycan analysis using lectin microarray</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t218" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t218</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|