Pyridylamino saccharides can be converted to the corresponding reducing sugar chains by two-step process. The first step is conversion to 1-amino-l-deoxy derivatives and the second is conversion to the corresponding reducing sugar chains using the Sommlet reaction. |
| Category | N-Glycans |
| Protocol Name | Conversion of Pyridylamino Sugar Chains to Corresponding Reducing Sugar Chains |
Authors
 |
Nakakita, Shin-ichi
*
Department of Functional Glycomics, Life Science Research Center, Kagawa University
Natsuka, Shunji
Department of Biology, Faculty of Science, Niigata University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Palladium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) |
| ● |
Hexamethylenetetramine (Wako Pure Chemical Industries Ltd.) |
| ● |
Hydrazine anhydrate (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) |
|
Instruments
 |
| ● |
Speed Vac Concentrator (Thermo Fisher Scientific Inc., Waltham, MA) |
| ● |
Block incubator (Astec Co., Ltd., Fukuoka, Japan) |
| ● |
Rotary oil pump connected to cold trap (Yamato Scientific Co., Ltd., Tokyo, Japan) |
| ● |
Hydrogen Generator (GL-Science, Tokyo, Japan) |
|
| Methods |
|
1. |
Preparation of 1-amino-1-deoxy derivative from pyridylamino saccharide |
| 1) |
Lyophilized pyridylamino saccharide (>1 nmol). |
Comment 0
|

|
| 2) |
Put on the bottom of a test tube. |
Comment 0
|

|
| 3) |
Add 1 mL of the 0.1% Acetic acid solution. |
Comment 0
|

|
| 6) |
Reduce with hydrogen gas at an atmospheric pressure and at room temperature for 3 h. |
Comment 0
|

|
| 7) |
Filtrate by Milex filter (0.22 μM). |
Comment 0
|

|
| 9) |
Add 0.2 mL of anhydrous hydrazine. |
Comment 0
|

|
| 10) |
Heated in a sealed tube at 70°C for 2 min. |
Comment 0
|

|
| 11) |
Remove excess hydrazine in vacuo. |
Comment 0
|

|
| 12) |
Gel filtration on a Bio-Gel P-2 column (1.2 × 90 cm; 10 mM acetic acid). |
Comment 0
|

|
| 13) |
Concentrate Ninhydrin positive fractions and lyophilized. |
Comment 0
|
|
|
|
2. |
Conversion of 1-amino-l-deoxy derivative to reducing saccharide |
| 1) |
1-amino-1-deoxy derivative (about 1 nmol/ 5 μL of water) is put on the bottom of a test tube. |
Comment 0
|

|
| 2) |
Add 8 μL of saturated hexamethylenetetramine aqueous solution. |
Comment 0
|

|
| 3) |
Add 1.5 μL of 50% acetic acid aqueous solution (pH of the mixed reaction solution is 4.5). |
Comment 0
|

|
| 4) |
Heat in a sealed tube at 100°C for 45 min. |
Comment 0
|

|
| 6) |
Concentrate to about 20 μL with a Speed Vac concentrator to remove the volatile reagents. |
Comment 0
|

|
| 8) |
Apply onto a TOYOPAK ODS cartridge. |
Comment 0
|

|
| 11) |
Add 0.6 g of Dowex 50W X2 (H+). |
Comment 0
|

|
| 12) |
Pour into a small glass column. |
Comment 0
|

|
| 13) |
Wash with 3 column volumes of water. |
Comment 0
|

|
| 14) |
Collect eluate and washings, and concentrated, and freeze-dried. |
Comment 0
|
|
|
| Initial amount | |
| Produced amount | |
| Discussion | This method clearly indicates that 1-amino-l-deoxy sugars can be converted to the corresponding sugar chains with reducing power by the Sommlet reaction. |
| Figure & Legends |
Figure & Legends

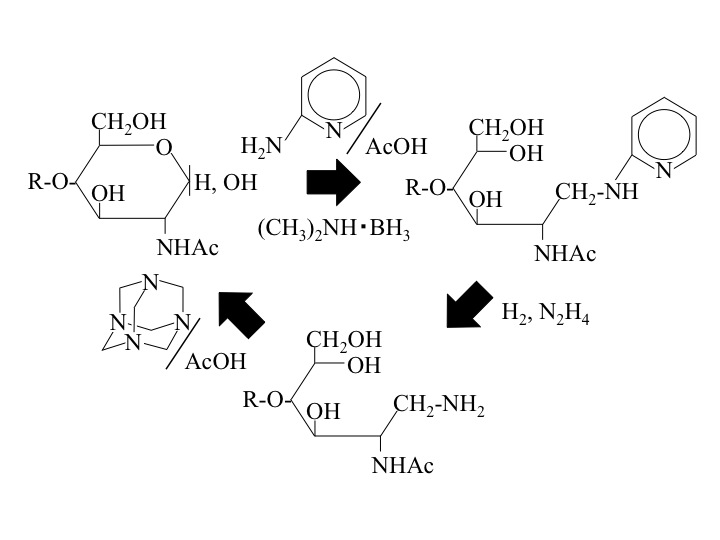
Fig. 1. Scheme for the conversion of a PA-sugar chain into a free sugar chain.
This figure was originally published in J Biochem. 134(1): 51–5 2003 " Conversion of pyridylamino sugar chains to corresponding reducing sugar chains" Takahashi C. Nakakita S. et al. Oxford Journals. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2015-05-08 15:03:46 |
- Takahashi, C., Nakakita, S., and Hase, S. (2003) Conversion of pyridylamino sugar chains to corresponding reducing sugar chains. J Biochem. 134, 51–5 [PMID : 12944370]
- Sommlet, M. (1913) Sur un mode de decomposition des halogenoalcoylates d' hexamethylene-tetramine. Compt. rend. 157, 852–854.
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Nakakita, Shin-ichi,
Natsuka, Shunji,
(2015). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.25,4,2024 .
How to Cite this Work in Website:
Nakakita, Shin-ichi,
Natsuka, Shunji,
(2015).
Conversion of Pyridylamino Sugar Chains to Corresponding Reducing Sugar Chains.
Retrieved 25,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t215.
html source
Nakakita, Shin-ichi,
Natsuka, Shunji,
(2015).
<b>Conversion of Pyridylamino Sugar Chains to Corresponding Reducing Sugar Chains</b>.
Retrieved 4 25,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t215" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t215</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|