The catalytic reaction of some endo-β-N-acetylglucosaminidases (endo-β-GlcNAc-ases), which hydrolyze the N,N’-diacetylchitobiose (GlcNAcβ1-4GlcNAc) moiety in oligosaccharides bound to the asparagine residue of various glycoproteins and glycopeptides through an N-glycosidic linkage, proceeds through a substrate-assisted mechanism via the participation of the 2-acetamido group. This reaction mechanism is analogous to the catalytic mechanisms of some glycoside hydrolase (GH) family 18 chitinases and some GH family 20 N-acetylhexosaminidases. These endo-β-GlcNAc-ases belong to GH family 85 and are assumed to catalyze hydrolysis and transglycosylation via an oxazolinium ion intermediate. Endo-M (endo-β-GlcNAc-ase from Mucor hiemalis) and Endo-A (endo-β-GlcNAc-ase from Arthrobacter protophormiae) accept synthetic oxazoline derived from Manβ1-4GlcNAc as a donor substrate for transglycosylation (1). The sugar oxazolines can serve as excellent donor substrates because of their highly activated nature and their ability to mimic the transition state, and also are useful activated glycosyl donors for GH family 85 enzymes (2). Recently, researchers have synthesized various sugar oxazolines and tested their activity for transglycosylation with GH 85 endo-β-GlcNAc-ases including Endo-M. Because Endo-M is inherently a glycoside hydrolase, a broader application of the enzymatic transglycosylation activity has been hampered by the issue of product hydrolysis resulting in low yield. Recently, the glycosynthase-like mutant, Endo-M-N175Q, which was successfully developed by site-directed mutagenesis of a key catalytic residue of the enzyme, exhibited highly efficient transglycosylation using a synthetic sugar oxazoline as a donor substrate (2, 3). Herein, we describe a novel and simple method to synthesize a sialo-complex type sugar oxazoline derived from a natural N-glycan by a one-pot chemical reaction using 2-chloro-1,3-dimethyl-2-imidazolinium chloride without any protection and deprotection steps (Title1) (4, 5). A conventional method to synthesize a high-mannose type sugar oxazoline is also described (Title2) (2). |
| Category | N-Glycans |
| Protocol Name | Synthesis of sugar oxazolines derived from natural N-glycan as substrates for transglycosylation reaction by Endo-M |
Authors
 |
Umekawa, Midori
College of Life Science, Ritsumeikan University
Yamamoto, Kenji
Research Institute for Bioresources and Biotechnology, Ishikawa Prefectural University
|
| KeyWords |
|
Reagents
 |
| ● |
Sialoglycopeptide from egg yolk (Taiyo Kagaku Co., Ltd., Yokkaichi, Japan) |
| ● |
2-Chloro-1,3-dimethyl-2-imidazolinium chloride (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) |
| ● |
Man9GlcNAc2Asn from soybean flour (Sigma-Aldrich, St. Louis, MO) |
| ● |
|
|
| Methods |
|
1. |
Synthesis and purification of a sialo-complex-type sugar oxazoline (Fig. 1) |
| 1) |
Sialoglycopeptide (SGP) from egg yolk (500 mg, final conc. 5 mM) was subjected to hydrolysis by Mucor hiemalis endo-β-N-acetylglucosaminidase, Endo-M (50 μg), in 50 mM sodium phosphate buffer (pH6.5) at 30˚C overnight. The reaction was stopped by heating at 100˚C for 5 min. |
Comment 1
|

|
| 2) |
The reaction mixture was applied to a Sephadex G-25 gel filtration column and eluted with ionized water. The fractions containing a sialo-complex-type glycan with a single GlcNAc at the reducing end (SG-GlcNAc) were collected and freeze dried (150 mg, -80% yield). |
Comment 1
|

|
| 3) |
The SG-GlcNAc (150 mg, final conc. 50 mM, 1eq.) was dissolved in 1.5 mL water and reacted with 15 eq. of 2-chloro-1,3-dimethyl-2-imidazolinium chloride (DMC) in the presence of 45 eq. of triethylamine on ice for 0.5–1h. The GlcNAc residue at the reducing end of SG-GlcNAc was almost completely converted to the sugar oxazoline derivative. |
Comment 0
|

|
| 4) |
The reaction mixture was applied to a Sephadex G-25 gel filtration column in 0.01% aqueous ammonia and the sialo-complex-type sugar oxazoline was obtained in 63% yield (freeze dried, 94.5 mg, the overall yield from SGP was 50% ). |
Comment 1
|
|
|
|
2. |
Synthesis and purification of a high-mannose-type sugar oxazoline (Fig. 2) |
| 1) |
The Man9GlcNAc2Asn (75 mg) was treated with immobilized Arthrobactor protophormiae endo-β-N-acetylglucosaminidase (Endo-A) in acetate buffer (pH 6.0). Subsequent gel filtration on a column of Sephadex G-25 gave pure Man9GlcNAc (45 mg). |
Comment 1
|

|
| 2) |
The oligosaccharide Man9GlcNAc (45 mg, 26.8 μmol) was dissolved in a mixture of pyridine (3 mL) and acetic anhydride (3 mL), and the solution was stirred at room temperature for 20 h. |
Comment 1
|

|
| 3) |
The freeze dried mixture was subjected to column chromatography on silica gel followed by elution with dichloromethane/methanol (20:1) to afford the fully acetylated Man9GlcNAc as a pale yellow syrup (56 mg). |
Comment 0
|

|
| 4) |
The acetylated Man9GlcNAc (56 mg) was dissolved in anhydrous 1,2-dichloroethane (4 mL), and then trimethylsilyl bromide (40 μL, 0.33 mmol), boron trifluoride etherate (40 μL, 0.33 mmol), and 2,4,6-collidine (47 μL, 0.33 mmol) were added sequentially under an argon atmosphere. The mixture was stirred at room temperature overnight. |
Comment 0
|

|
| 5) |
The solution was diluted with chloroform (20 mL) and washed with saturated sodium bicarbonate solution and brine. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated. |
Comment 0
|

|
| 6) |
The residue was purified by column chromatography on silica gel and eluted with dichloromethane/methanol (20:1). The crude yellow solid was further purified by gel filtration (Sephadex LH-20, eluted with methanol) to afford the acetylated Man9GlcNAc-oxazoline derivative as a pale yellow foam (40 mg). |
Comment 1
|

|
| 7) |
The acetylated Man9GlcNAc-oxazoline (40 mg, 13.7 mmol) was treated with sodium methoxide in anhydrous methanol (4 mL) for 16 h, and the de-O-acetylation was monitored by ESI-MS. The methanol was removed by evaporation. |
Comment 0
|

|
| 8) |
The residue was dissolved in water and subjected to gel filtration (Sephadex G-10, eluted with water containing 0.03% triethylamine). The fractions containing the product were collected and freeze dried. The Man9GlcNAc-oxazoline was obtained as a pale yellow solid (24 mg). |
Comment 1
|
|
|
| Initial amount | Title1-1) 500 mg
Title2-1) 75 mg |
| Produced amount | Title1-4) 94.5 mg
Title1-7) 24 mg |
| Figure & Legends |
Figure & Legends

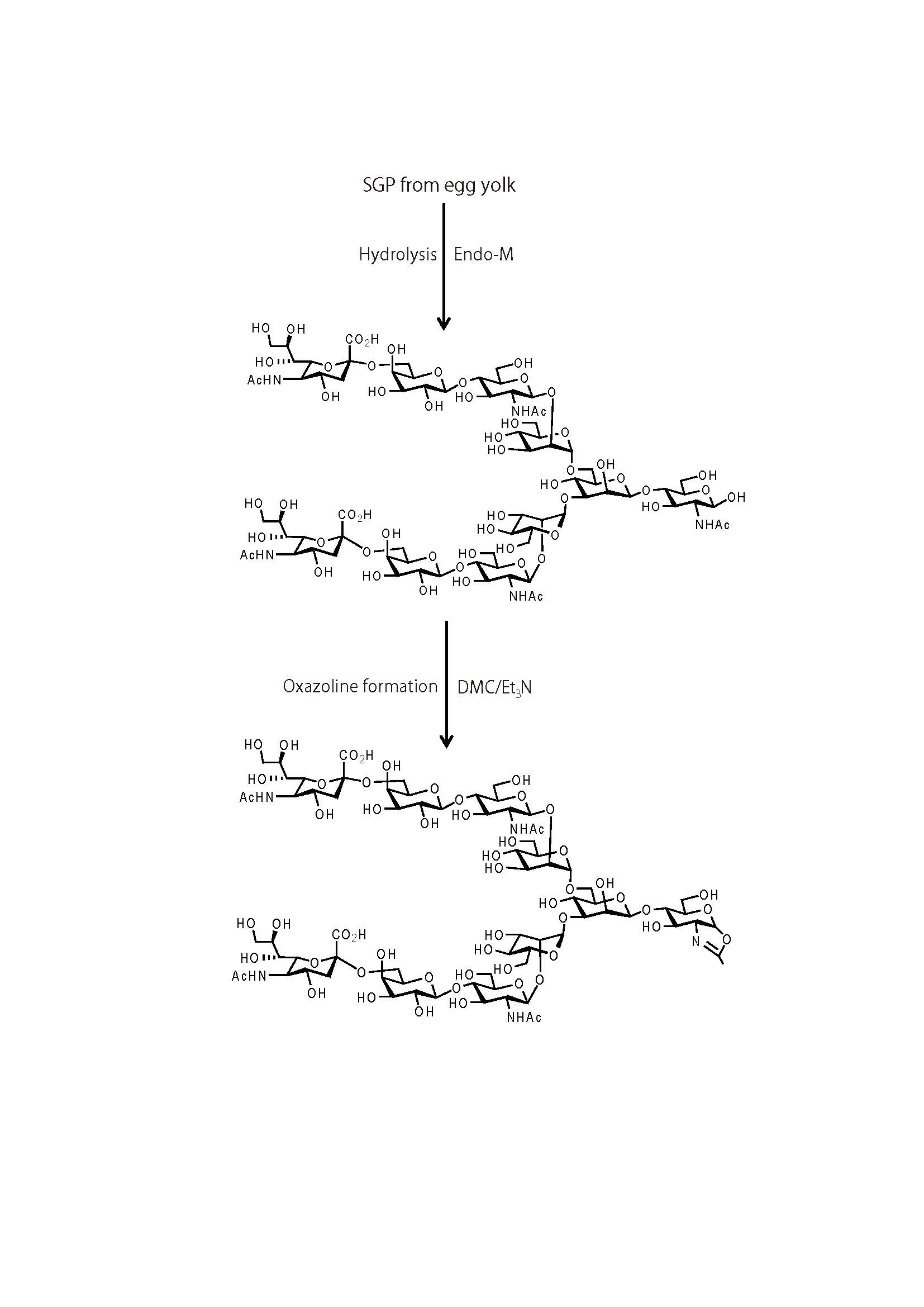
Fig. 1. Synthesis of sialo-complex-type sugar oxazoline.
SG-GlcNAc, decasaccharide of sialo-complex-type GlcNAc; DMC, 2-chloro-1,3-dimethyl-2-imidazolinium chloride; Et3N, triethylamine
This figure was originally published in Biochim Biophys Acta. Umekawa M. et al. "Efficient transfer of sialo-oligosaccharide onto proteins by combined use of a gycosynthase-like mutant of Mucor hiemalis endoglycosidase and synthetic sialo-complex-type sugar oxazoline" 2010, 1800(11): 1203-9.

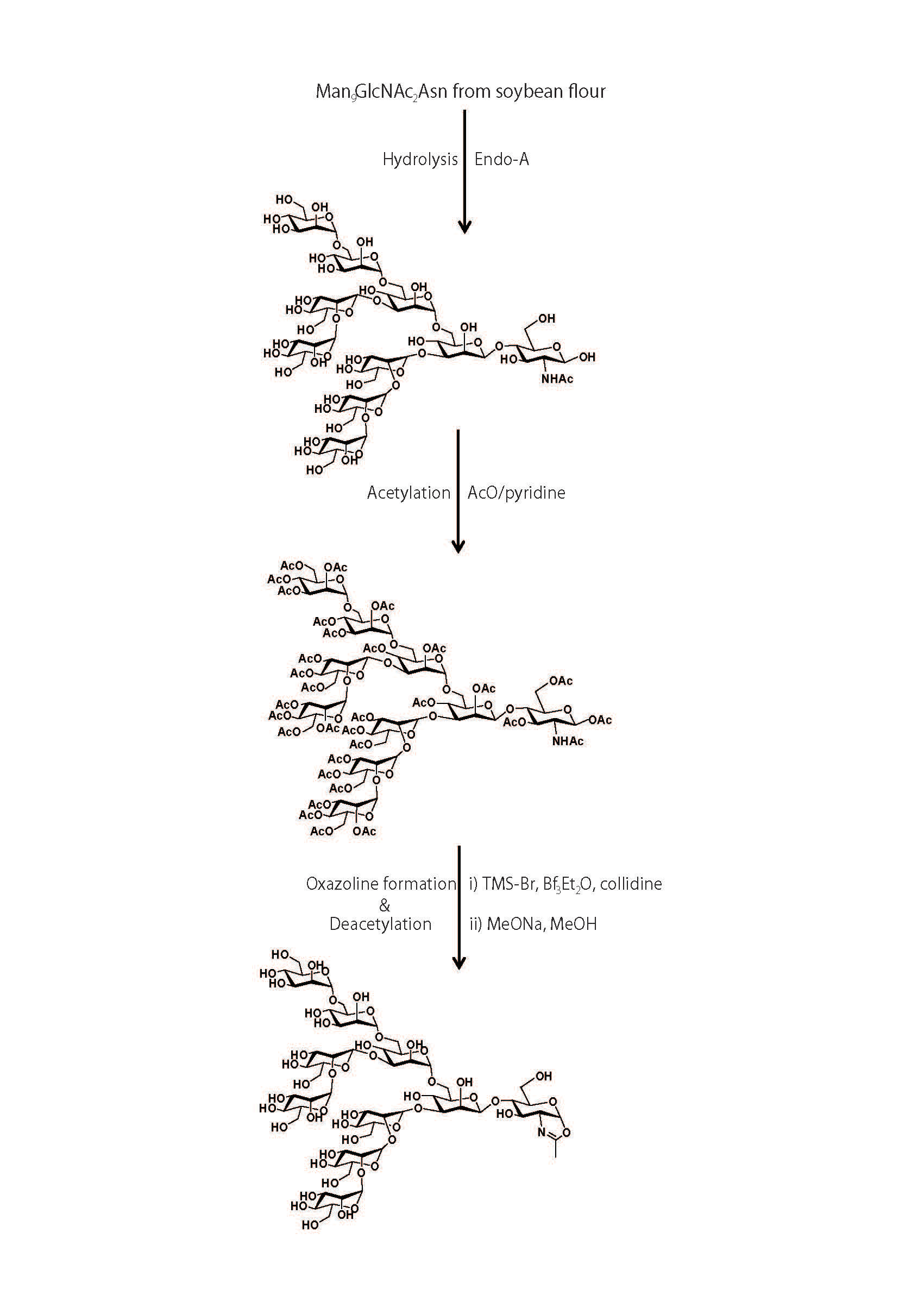
Fig. 2. Synthesis of high-mannose-type sugar oxazoline.
AcO, acetic anhydride; TMS-Br, trimethylsilyl bromide; Bf3Et2O, boron trifluoride etherate; MeONa, sodium methoxide; MeOH, methanol
This figure was originally published in J Biol Chem. Umekawa M. et al. "Mutants of Mucor hiemalis endo-beta-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activivites" 2008, 283(8): 4469-79. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-05-28 09:26:28 |
- Fujita, M., Shoda, S., Haneda, K., Inazu, T., Takegawa, K., Yamamoto, K. (2001) A novel disaccharide substrate having 1,2-oxazoline moiety for detection of transglycosylating activity of endoglycosidases. Biochim Biophys Acta. 1528, 9-14 [PMID : 11514092]
- Umekawa, M., Huang, W., Li, B., Fujita, K., Ashida, H., Wang, L.X., Yamamoto, K. (2008) Mutants of Mucor hiemalis endo-beta-N-acetylglucosaminidase show enhanced transglycosylation and glycosynthase-like activities. J Biol Chem. 283, 4469-4479 [PMID : 18096701]
- Umekawa, M., Li, C., Higashiyama, T., Huang, W., Ashida, H., Yamamoto, K., Wang, L.X. (2010) Efficient glycosynthase mutant derived from Mucor hiemalis endo-beta-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J Biol Chem. 285, 511-521 [PMID : 19880511]
- Noguchi, M., Tanaka, T., Gyakushi, H., Kobayashi, A., Shoda, S. (2009) Efficient synthesis of sugar oxazolines from unprotected N-acetyl-2-amino sugars by using chloroformamidinium reagent in water. J Org Chem. 74, 2210-2212 [PMID : 19203234]
- Umekawa, M., Higashiyama, T., Koga, Y., Tanaka, T., Noguchi, M., Kobayashi, A., Shoda, S., Huang, W., Wang, LX., Ashida, H., Yamamoto, K. (2010) Efficient transfer of sialo-oligosaccharide onto proteins by combined use of a glycosynthase-like mutant of Mucor hiemalis endoglycosidase and synthetic sialo-complex-type sugar oxazoline. Biochim Biophys Acta. 1800, 1203-1209 [PMID : 20647032]
- Wang, L.X., Ni, J., Singh, S., Li, H. (2004) Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: implications for HIV-1 vaccine design. Chem Biol. 11, 127-134 [PMID : 15113002]
- Fujita, K., Tanaka, N., Sano, M., Kato, I., Asada, Y., Takegawa, K. (2000) Synthesis of neoglycoenzymes with homogeneous N-linked oligosaccharides using immobilized endo-beta-N-acetylglucosaminidase A. Biochem Biophys Res Commun. 267, 134-138 [PMID : 10623587]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Umekawa, Midori,
Yamamoto, Kenji,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.24,4,2024 .
How to Cite this Work in Website:
Umekawa, Midori,
Yamamoto, Kenji,
(2014).
Synthesis of sugar oxazolines derived from natural N-glycan as substrates for transglycosylation reaction by Endo-M.
Retrieved 24,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t208.
html source
Umekawa, Midori,
Yamamoto, Kenji,
(2014).
<b>Synthesis of sugar oxazolines derived from natural N-glycan as substrates for transglycosylation reaction by Endo-M</b>.
Retrieved 4 24,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t208" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t208</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|