Animal models of intestinal inflammation are indispensable for understanding of the pathogenesis of Crohn’s disease (CD) and ulcerative colitis (UC), the two major forms of inflammatory bowel disease (IBD) in humans. Although these two diseases have certain characteristics in common, most patients can be clearly diagnosed as CD or UC based on important clinical differences. UC primarily affects the mucosal layer of the colon and rectum, whereas CD usually affects the entire intestinal wall and may extend to any part of the gastrointestinal tract. The etiology of both CD and UC remains unclear. However, in recent years, epidemiologic and genetic studies in humans and particularly in IBD-related animal models suggested that the combination of genetic susceptibility and altered immune responses driven by microbial factors in the enteric environment contributes to initiation and chronification of these diseases. In this protocol, we describe and analyze the methods for the most widely used chemically induced models of intestinal inflammation, murine 2,4,6-trinitro benzene sulfonic acid (TNBS)-, oxazolone- and acute dextran sodium sulfate (DSS)-induced colitis. TNBS and oxazolone are believed to induce a T-cell-mediated response against hapten-modified autologous proteins/luminal antigens, whereas DSS appears to be directly toxic to colonic epithelial cells of the basal crypts. Although these models possess limitations like other animal models, they mimic some important immunological and histopathological aspects of human IBD. |
| Category | Biosynthesis & Metabolism |
| Protocol Name | Induction of Colitis and its analyses |
Authors
 |
Ishii, Mayuko
Department of Molecular Biochemistry and Clinical Investigation, Osaka University Graduate School of Medicine
Shinzaki, Shinichiro
Department of Gastroenterology and Hepatology, Osaka University Graduate School of Medicine
Miyoshi, Eiji
Department of Molecular Biochemistry and Clinical Investigation, Osaka University Graduate School of Medicine
|
| KeyWords |
|
Reagents
 |
| ● |
Gender- and age-matched mice, 8 to 12 weeks old |
| ● |
DSS salt (molecular weight: 36,000–50,000) |
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
Autoclaved drinking water |
| ● |
Neutral buffered 10% formalin |
| ● |
RPMI 1640 cell culture medium |
| ● |
|
| ● |
|
| ● |
|
|
Instruments
 |
| ● |
|
| ● |
Electronic weighing instrument |
| ● |
|
| ● |
MAS-coated slide (Matsunami Glass Ind., Ltd., Osaka, Japan, #S9941) |
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
|
| Methods |
|
1. |
Induction of colitis (A) TNBS-induced colitis |
| 1) |
On day 1, carefully shave a 2 × 2 cm field of the skin of the mouse using a razor under anesthesia with tribromoethanol. |
Comment 0
|

|
| 2) |
Sensitize with 150 μL haptenating agent TNBS at a concentration of 2.5% in 50% ethanol by skin painting using a 200 μL pipette. |
Comment 0
|

|
| 3) |
On day 8, administer intrarectally 6 mL/kg of 1% TNBS in 50% ethanol via a 3.5F catheter under anesthesia with tribromoethanol. |
Comment 1
|

|
| 4) |
Remove the catheter gently from the colon and keep the mouse with the head down in a vertical position for 1 min. |
Comment 1
|

|
| 6) |
On day 12, sacrifice the mice and analyze.
◇ Weigh the mice every day from day 8 to day 12 (sacrifice). |
Comment 0
|
|
|
|
2. |
Induction of colitis (B) Oxazolone-induced colitis |
| 1) |
On day 1, carefully shave a 2 × 2 cm field of the skin of the mouse using a razor under anesthesia with tribromoethanol. |
Comment 0
|

|
| 2) |
Sensitize with 150 μL haptenating agent oxazolone at a concentration of 3% in 100% ethanol by skin painting using a 200 μL pipette |
Comment 0
|

|
| 3) |
On day 8, administer intrarectally 6 mL/kg of 1% oxazolone in 50% ethanol via a 3.5F catheter under anesthesia with tribromoethanol. |
Comment 1
|

|
| 4) |
Remove the catheter gently from the colon and keep the mouse with the head down in a vertical position for 1 min. |
Comment 1
|

|
| 6) |
On day 12, sacrifice the mice and analyze.
◇ Weigh the mice every day from day 8 to day 12 (sacrifice). |
Comment 0
|
|
|
|
3. |
Induction of colitis (C) Acute DSS-induced colitis |
| 1) |
On day 1, add DSS to the drinking water (autoclaved water) at the concentration of 3%.
◇ Weigh the mice every day from day 1 to day 10 (sacrifice). |
Comment 0
|

|
| 2) |
On day 8, replace the remaining DSS solution with autoclaved water. |
Comment 0
|

|
| 3) |
On day 10, sacrifice the mice and analyze. |
Comment 0
|
|
|
|
4. |
Analysis of colitis (A) Weight |
| 1) |
Weigh each mouse every day from day 8 (TNBS- or oxazolone-induced colitis) or day 1 (DSS-induced colitis) to sacrifice. |
Comment 0
|
|
|
|
5. |
Analysis of colitis (B) Colon length |
| 1) |
Separate the colon and measure the length between the ileocecal junction and anus. |
Comment 0
|
|
|
|
6. |
Analysis of colitis (C) Isolation of mesenteric lymph node (MLN) cells |
| 1) |
Localize and remove MLNs aseptically. |
Comment 0
|

|
| 2) |
Make a single-cell suspension by pressing through a 40 μm cell strainer positioned on a 50 mL Falcon tube using the plunger of a 1 mL syringe. |
Comment 0
|

|
| 3) |
Rinse the cell strainer with 10 mL RPMI cell culture medium containing 2% FCS. |
Comment 0
|

|
| 4) |
Pellet cells by centrifugation for 5 min (1,500 rpm, 20°C). |
Comment 0
|

|
| 5) |
Discard the supernatant, and resuspend cells in 10 mL of RPMI medium. |
Comment 0
|

|
| 6) |
Pellet cells by centrifugation for 5 min (1,500 rpm, 20°C). |
Comment 0
|

|
| 7) |
Discard the supernatant, resuspend cells in 2 mL RPMI medium and count cells for subsequent analysis. |
Comment 0
|
|
|
|
7. |
Analysis of colitis (D) Histological evaluation of colitis severity |
| 1) |
Cut defined 0.5-cm-long colonic fragments from proximal and distal parts of the colon. |
Comment 0
|

|
| 2) |
Immerse immediately in neutral buffered 10% formalin and incubate overnight. |
Comment 0
|

|
| 3) |
Prepare 5 mm paraffin cross-sections. |
Comment 0
|

|
| 4) |
Stain sections with hematoxylin and eosin using appropriate standard procedures. |
Comment 0
|

|
| 5) |
Determine the histological score by summing the score of injury and infiltration in proximal and distal parts of the colon. Various scoring systems are reported, and a representative system is shown in TABLES 1 (3), 2 (4), and 3 (4). |
Comment 0
|
|
|
| Discussion | The clinical appearance of human IBD is heterogeneous, a fact that is also reflected by the steadily increasing number of transgenic or gene-targeted mouse strains displaying IBD-like intestinal alterations. When chosen appropriately, these models can be used to investigate pathophysiological mechanisms and test emerging therapeutic strategies in the preclinical phase. Because the onset of inflammation is immediate and the procedure is relatively straightforward, chemically induced models of intestinal inflammation belong to the most commonly used animal IBD models.
TNBS-induced colitis is a Th1 T-cell-mediated colitis that captures many of the features of Crohn’s disease, which is characterized by a transmural, granulomatous inflammation occurring anywhere in the alimentary canal but usually centered in the terminal ileum and ascending colon (5). Conversely, oxazolone-induced colitis is associated with an atypical Th2-mediated response characterized by secretion of interleukin-13 and captures many of the features of ulcerative colitis (6). |
| Figure & Legends |
Figure & Legends

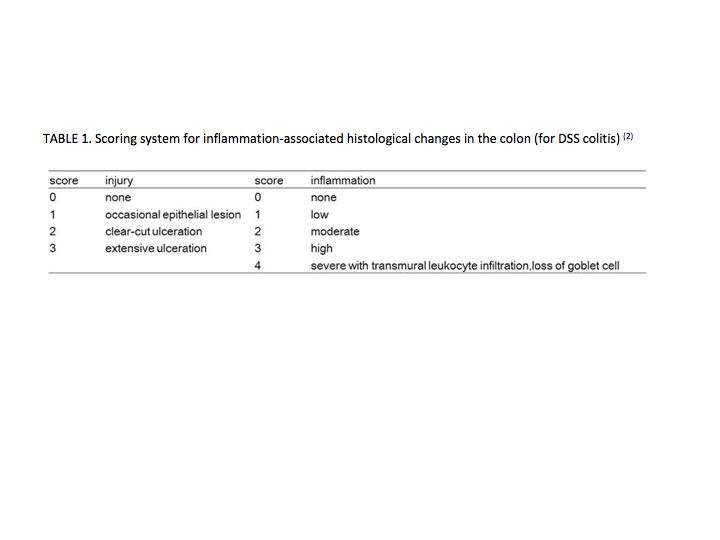



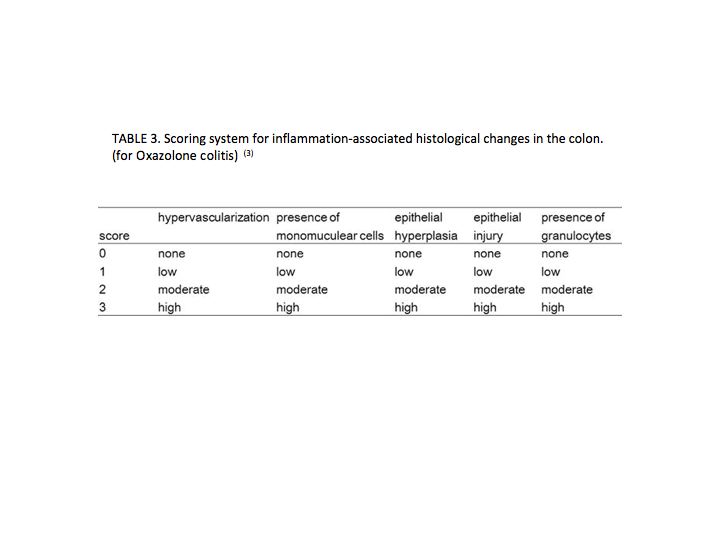

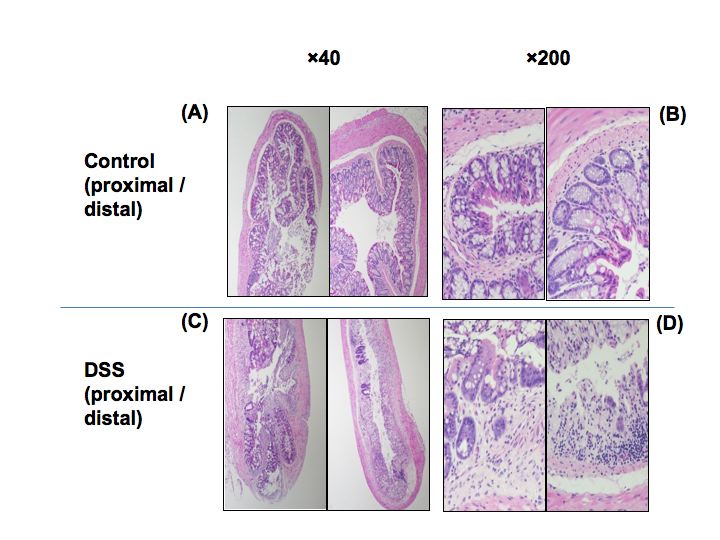
Fig. 1. Photographs of hematoxylin and eosin-stained colon cross-sections in mice with colitis subjected to DSS on day 10.
(A and B) Control mice. (C and D) DSS mice. Left, proximal part. Right, distal part. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-31 09:29:52 |
- Stefan Wirtz. (2007) Chemically induced mouse models of intestinal inflammation. Nature Protocols. 2, 541–546 [PMID : 17406617]
- Shinzaki S. (2012) Altered oligosaccharide structures reduce colitis induction in mice defective in β-1,4-galactosyltransferase. Gastroenterology. 142, 1172–82 [PMID : 22333949]
- Dohi T. (2005) Therapeutic potential of follistatin for colonic inflammation in mice. Gastroenterology. 128, 411–23 [PMID : 15685552]
- Iijima H. (2004) Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 199, 471–82 [PMID : 14970176]
- Stefan Fichtner-Feigl. (2005) Treatment of murine Th1- and Th2- mediated inflammatory bowel disease with NF-κB decoy oligonucleotides. The Journal of Clinical Investigation. 115, 3057–3071 [PMID : 16239967]
- Frank Heller. (2002) Oxazolone Colitis, a Th2 Colitis Model Resembling Ulcerative Colitis, Is Mediated by L-13-Producing NK-T Cells. Immunity. 17, 629–638 [PMID : 12433369]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Ishii, Mayuko,
Shinzaki, Shinichiro,
Miyoshi, Eiji,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Ishii, Mayuko,
Shinzaki, Shinichiro,
Miyoshi, Eiji,
(2014).
Induction of Colitis and its analyses.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t207.
html source
Ishii, Mayuko,
Shinzaki, Shinichiro,
Miyoshi, Eiji,
(2014).
<b>Induction of Colitis and its analyses</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t207" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t207</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|