Dendritic spines are highly specialized actin-rich small protrusions on neuronal dendrites and act as receptive sites for excitatory synaptic transmission. It is widely believed that long-term synaptic plasticity is accompanied by structural changes in synapses, particularly at dendritic spines. Therefore, adequate visualization of dendritic spines is critically important in order to study synaptic plasticity. We described here a method employing a lipophilic fluorescent dye, DiI (1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate), for visualization of the fine structures of spines on dendrites. |
| Category | Glycogene transgenic animals |
| Protocol Name | |
Authors
 |
Morise, Jyoji
Department of Biological Chemistry, Human Health Sciences, Graduate School of Medicine, Kyoto University
Morita, Ippei
Department of Biological Chemistry, Graduate School of Pharmaceutical Sciences, Kyoto University
Oka, Shogo
*
Department of Biological Chemistry, Human Health Sciences, Graduate School of Medicine, Kyoto University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (Invitrogen/Life Technologies, Carlsbad, CA) |
| ● |
Phosphate-buffered saline (PBS) |
| ● |
4% (or 2%) Paraformaldehyde (PFA) in PBS |
| ● |
ProLong Gold antifade reagent (Invitrogen/Life Technologies, Carlsbad, CA) |
|
Instruments
 |
| ● |
LEICA VT1000S (Leica Microsystems GmbH, Watzlar, Germany) |
| ● |
|
| ● |
PC-10 Micropipette-puller (Narishige Co., Ltd., Tokyo, Japan) |
| ● |
GD-1 Glass Capillary with Filament 1x90mm (Narishige Co., Ltd.,Tokyo, Japan) |
| ● |
MN-151 Joystick Micromanipulator (Narishige Co., Ltd.,Tokyo, Japan) |
| ● |
Micro Slide Glass Superfrost (Matsunami Glass Ind., Ltd., Osaka, Japan) |
| ● |
Micro Cover Glass 24x24mm Thickness 0.12–0.17mm (Matsunami Glass Ind., Ltd.) |
| ● |
24-well Multiwell Plate (BD, Franklin Lakes, NJ) |
| ● |
10-cm Petri-dish (BD, Franklin Lakes, NJ) |
| ● |
|
| ● |
|
| ● |
|
|
| Methods |
|
1. |
Preparation of a mouse brain |
| 1) |
Deeply anesthetize a mouse with pentobarbital and perfused with 4% paraformaldehyde (PFA) in PBS. |
Comment 0
|

|
| 2) |
Excise the whole brain of the mouse. |
Comment 0
|

|
| 3) |
Immerse the whole brain in 4% paraformaldehyde (PFA) in PBS for 2 days. |
Comment 0
|

|
| 4) |
Separate the brain into right and left hemispheres along the sagittal plane (see Fig. 1(1)) with a knife blade. |
Comment 0
|

|
| 5) |
Cut off the olfactory bulb and cerebellum in the coronal plane (see Fig. 1(2) and (3), respectively). |
Comment 0
|
|
|
|
2. |
Preparation of a hippocampal brain slice |
| 1) |
Set the brain on the stage of a LEICA VT1000S as shown in Fig. 1(4). |
Comment 0
|

|
| 2) |
Prepare coronal sections of the brain (100 μm thick) including the hippocampal region according to the manufacture’s protocol of LEICA VT1000S. |
Comment 1
|

|
| 3) |
Pick up each slice with a brush and put into wells of a 24-well Multiwell Plate filled with PBS. |
Comment 0
|

|
| 4) |
Move an appropriate slice from 24-well Multiwell Plate to 10-cm Petri-dish filled with PBS. |
Comment 0
|

|
| 5) |
Transfer the slice onto a Micro Slide Glass with a brush. |
Comment 0
|
|
|
|
3. |
|
| 1) |
After preparation of a glass capillary with Micropipette-puller, set the capillary on the Joystick Micromanipulator. |
Comment 0
|

|
| 2) |
Finely crush DiI crystals and attach the DiI powder to the surface of the capillary tip. |
Comment 0
|

|
| 3) |
Observing with a stereoscopic microscope, deliver the DiI to several parts of pyramidal cell layer in the hippocampal CA1 region by lightly touching or gently poking the slice with the capillary (Fig. 2a). |
Comment 1
|

|
| 4) |
Move the dye-labeled slice from the slide to a 24-well Multiwell Plate filled with 4% (or 2%) PFA and keep in the shade for 24 to 48 h. |
Comment 0
|

|
| 5) |
Move the slice to a 10-cm Petri-dish filled with PBS. After transferring the slice onto a Micro Slide Glass, dry the slice. |
Comment 0
|

|
| 6) |
Mount the slice with 20 µL of ProLong Gold antifade reagent and cover it with a Micro Cover Glass. |
Comment 0
|

|
| 7) |
Observe the dye-labeled pyramidal cells with a fluorescence microscope. |
Comment 0
|
|
|
|
4. |
Measurement of DiI-labeled spines |
| 1) |
Capture some images of dye-labeled apical dendrites (Fig. 2b), and measure the length and width of spines along the dendrites using image analysis software (Fig. 2c). |
Comment 0
|

|
| 2) |
Count spine density as the number of spines per 10 μm dendrite. |
Comment 0
|

|
| 3) |
Classify dendritic spines as follows: mature spines are defined as 0.5–1.5 μm length spines with mushroom- like heads, and filopodium-like spines are longer than 1.5 μm protrusions without spine heads (see Fig. 2b) |
Comment 0
|
|
|
| Notes | Do not freeze the brain samples; keep them at 4℃.
The freeze-thaw process damages the membrane structure of spines. |
| Discussion | We applied this method to the functional analysis of HNK-1 glyco-epitope. GlcAT-P is a glucuronyltransferase, a key enzyme for the HNK-1 epitope biosynthesis. GlcAT-P-deficient mice showed an almost complete loss of HNK-1 expression in their brains and exhibited reduced LTP at Schaffer collateral-CA1 synapses along with defects in spatial memory formation. We then used this method to examine whether the loss of the HNK-1 epitope resulted in abnormalities in the neuronal fine structure. As a result, we found that GlcAT-P-deficient mice had a large number of filopodium-like immature spines on CA1 pyramidal neurons during the early postnatal period. Although there are several different views on the origin of dendritic spines, one model is that spines initially form as filopodium-like protrusions and that these structures are then converted directly to mature spines with mushroom-like heads. Therefore, impairment of the processes of spine maturation may cause reduced LTP in GlcAT-P deficient mice. The DiI labeling method is useful for other glycosyltransferase-gene-deficient mice which showed impaired synaptic plasticity in the nervous system, to investigate the molecular mechanism from a structural point of view. |
| Figure & Legends |
Figure & Legends

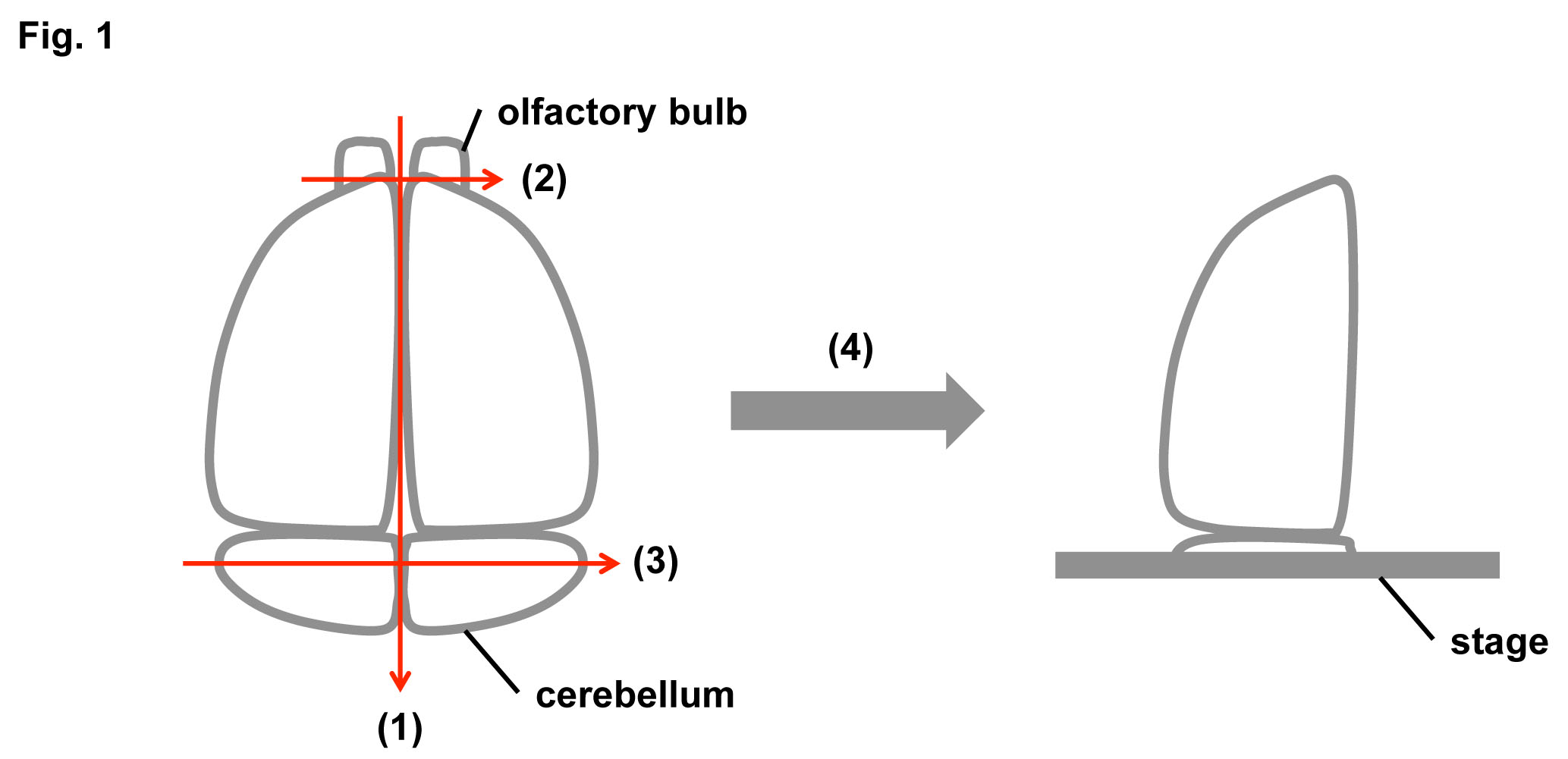
Fig. 1. Schematic diagrams of a mouse brain preparation
Separate the brain into right and left hemispheres along the sagittal plane (1). Cut off the olfactory bulb (2). Cut off the cerebellum in the coronal plane (3). Set the brain on the stage of a LEICA VT1000S (4).

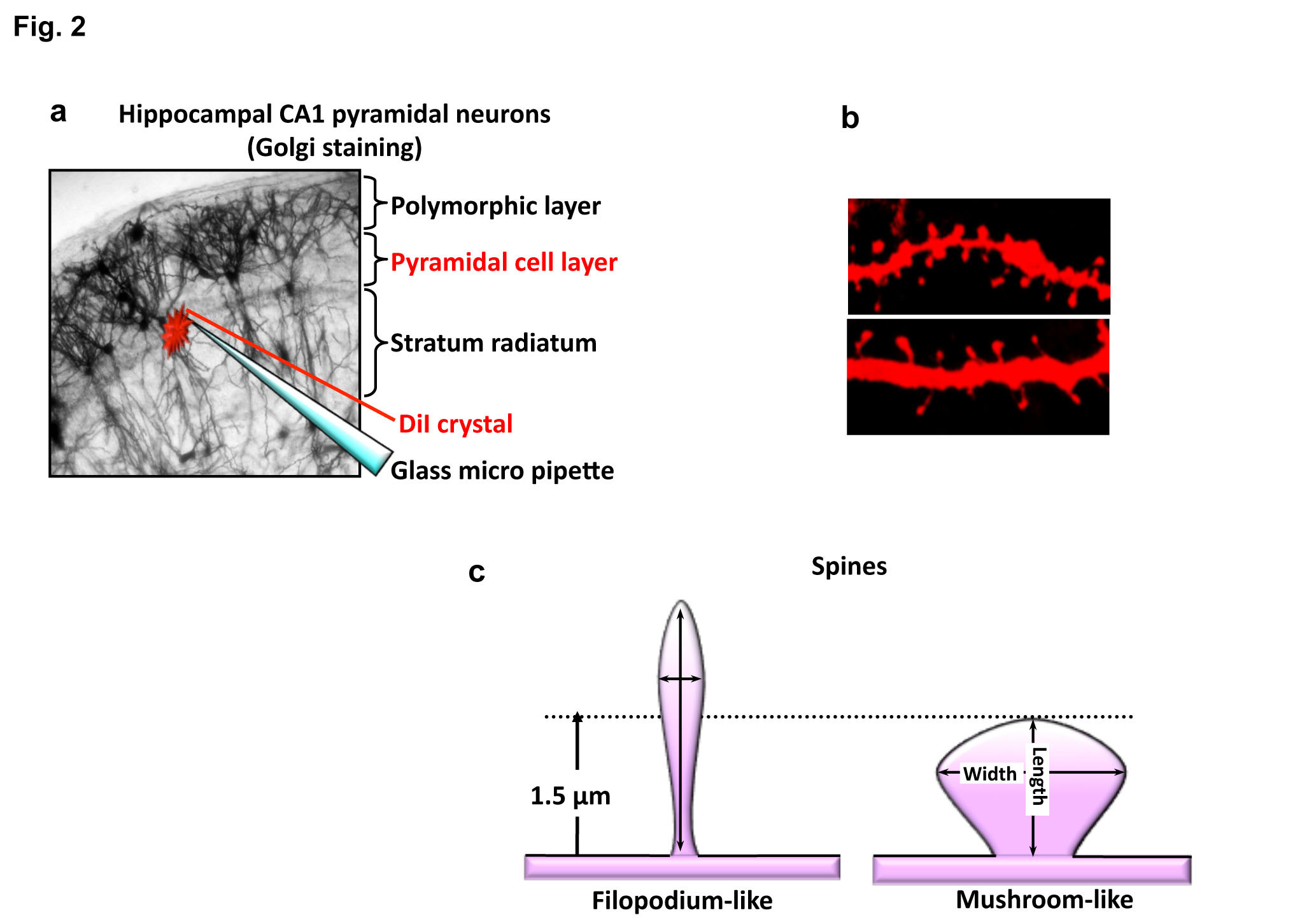
Fig. 2. Schematic diagrams and typical images of DiI labeling
a) Put the crushed DiI crystal on the pyramidal cell layer using a glass micro pipette.
b) Representative images of DiI-labeled dendritic spines.
c) Schematic diagrams of mushroom-like (mature type) and filopodium-like (immature type) spines |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-02-26 19:16:46 |
- Matsuno, H., Okabe, S., Mishina, M., Yanagida, T., Mori, K., and Yoshihara, Y. (2006) Telencephalin slows spine maturation. J. Neurosci. 26, 1776-1786 [PMID : 16467526]
- Morita, I., Kakuda, S., Takeuchi, Y., Kawasaki, T., Oka, S. (2009) HNK-1 (human natural killer-1) glyco-epitope is essential for normal spine morphogenesis in developing hippocampal neurons. Neuroscience. 164, 1685-94 [PMID : 19796667]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Morise, Jyoji,
Morita, Ippei,
Oka, Shogo,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.25,4,2024 .
How to Cite this Work in Website:
Morise, Jyoji,
Morita, Ippei,
Oka, Shogo,
(2014).
DiI Labeling experiments.
Retrieved 25,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t202.
html source
Morise, Jyoji,
Morita, Ippei,
Oka, Shogo,
(2014).
<b>DiI Labeling experiments</b>.
Retrieved 4 25,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t202" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t202</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|