Keratan sulfate is one of the major glycosaminoglycans and consists of poly-N-acetyllactosamine backbone glycans (→3Galβ1→4GlcNAcβ1→)n and sulfate residues at the 6-OH of Gal and GlcNAc residues. Based on the substrate specificities of glyco/sulfotransferases involved and in vivo overexpression and knockdown experiments, the biosynthetic pathway of keratan sulfate has been proposed as follows (Figure);
- Non-reducing terminal GlcNAc residues on specific branches of N-linked/O-linked glycans are sulfated at the 6-OH position by CHST6 (also known as GlcNAc 6-O-sulfotransferase-5, GlcNAc6ST5, CGn6ST, and GST4β).
- GlcNAc 6-O-sulfate moieties are next galactosylated at the 4-OH position by B4GALT4 (also known as β1,4-galactosyltransferase-IV, β4GalT-4, and β4GalT-IV), which is specific for GlcNAc 6-O-sulfate.
- 6-O-sulfated LacNAc, Galβ1→4(SO3-→6)GlcNAc, is elongated by B3GNT7 (also known as β1,3-N-acetylglucosaminyltransferase-7 and β3GnT7), which specifically recognizes sulfate residues.
- Repeating 1,2, and 3, resulting in the formation of [→3Galβ1→4(SO3-→6)GlcNAcβ1→]n polymer.
- CHST1 (also known as Gal 6-O-sulfotransferase, Gal6ST, and KS6ST) catalyzes sulfation at the 6-OH of Gal residues.
As described above, two glycosyltransferases, B4GALT4 and B3GNT7, are involved in the biosynthesis of keratan sulfate and its related glycans. The enzymatic activities of the two enzymes can be measured using with radiolabeled donor substrates and keratan sulfate-related glycans as acceptor substrates. |
| Category | Glycosyltransferases & related proteins |
| Protocol Name | Enzyme assay of GAG glycosyltransferases for keratan sulfate |
Authors
 |
Seko, Akira
Ito Glycotrilogy Project, Japan Science and Technology Agency (JST)
|
| KeyWords |
|
Reagents
 |
| ● |
UDP-[3H]galactose (0.555–1.11 TBq/mmol, American Radiolabeled Chemicals Inc., St. Louis, MO) |
| ● |
UDP-[3H]N-acetylglucosamine (0.74–1.66 TBq/mmol, PerkinElmer, Waltham, MA) |
|
Instruments
 |
| ● |
High-voltage paper electrophoresis unit (Advantec, Co., Ltd., Ehime, Japan) |
| ● |
Radiochromatogram scanner (RITA, Raytest, Straubenhardt, Germany) |
|
| Methods |
|
1. |
|
| 1) |
Twenty μL of the reaction mixture containing 50 mM HEPES-NaOH (pH 7.2), 10 mM MnCl2, 0.5 % (w/v) Triton X-100, 250 μM UDP-Gal, 0.3 μM UDP-[3H]Gal (4.9 × 105 dpm), 0.5 mM agL2L2 [SO3--6GlcNAcβ1-3Galβ1-4(SO3--6)GlcNAc], and the enzyme fraction, is incubated at 37°C for 1 h. |
Comment 0
|

|
| 2) |
Add 0.5 mL of 0.01 N HCl and heat at 100°C for 10 min to destroy residual UDP-[3H]Gal. After cooling, the mixture is neutralized with 1 N NaOH and concentrated by vacuum evaporator. |
Comment 0
|

|
| 3) |
Spot the mixture and bromophenol blue as a marker on a Whatman No. 1 paper (46 × 57 cm). Wet the paper with a solvent, pyridine/acetic acid/water=3:1:387 (pH 5.4). |
Comment 0
|

|
| 4) |
Set the paper into the high-voltage paper electrophoresis unit and perform electrophoresis using with the same solvent as 3), until bromophenol blue moves 10 cm from the origin. |
Comment 0
|

|
| 5) |
Dry the paper in the draft and measure the radioactivity using with a radiochromatogram scanner. The reaction product, [3H]Galb1-4agL2L2, moves approximately 10 cm from the origin. |
Comment 0
|
|
|
|
2. |
|
| 1) |
Twenty μL of the reaction mixture containing 50 mM HEPES-NaOH (pH 7.2), 10 mM MnCl2, 0.5 % (w/v) Triton X-100, 30 μM UDP-GlcNAc, 2.5 μM UDP-[3H]GlcNAc (6.7 × 106 dpm), 0.1 M GlcNAc, 1 mM AMP, 0.5 mM L2L2 [Galβ1-4(SO3--6)GlcNAcβ1-3Galβ1-4(SO3--6)GlcNAc](Ref. 3), and the enzyme fraction, is incubated at 37°C for 3 h. The enzymatic reaction is stopped by heating. |
Comment 0
|

|
| 2) |
Spot the mixture and bromophenol blue as a marker, on a Whatman No. 1 paper (46 × 57 cm). Wet the paper with a solvent, pyridine/acetic acid/water=3:1:387 (pH 5.4). |
Comment 0
|

|
| 3) |
Set the paper into the high-voltage paper electrophoresis unit and perform electrophoresis using with the same solvent as 3), until bromophenol blue moves 10 cm from the origin. |
Comment 0
|

|
| 4) |
Dry the paper in the draft and measure the radioactivity using with a radiochromatogram scanner. The reaction product, [3H]GlcNAcβ1-3L2L2, moves approximately 8.5 cm from the origin. |
Comment 0
|
|
|
| Notes |
- B4GALT4 belongs to B4GALT family which consists of 7 members in human. Among the members, only B4GALT4 acts efficiently on agL2L2. Other two enzymes, B4GALT1 and B4GALT3, exhibit very weak activities for agL2L2 (Ref. 1). If you would use crude fractions for enzymatic source, it would be recommended to distinguish B4GALT4 activity from B4GALT1 and B4GALT3 ones that you also measure galactosyltransferase activities for core2-O-pNP [Galβ1-3(GlcNAcβ1-6)GalNAcα1-p-nitrophenyl]. The relative values of the enzymatic activities for core2-O-pNP and agL2L2 are 102:3 (B4GALT1), 100:3 (B4GALT3), and 27:144 (B4GALT4).
- Similarly, B3GNT7 belongs to B3GNT/B3GALT large family. So far, eight B3GNT genes have been identified in human. Among them, three enzymes, B3GNT1, B3GNT2, and B3GNT7 recognize L2L2 as an acceptor substrate, however, only B3GNT7 acts efficiently on L2L2 (Ref. 2). The relative values of the enzymatic activities for LNnT (Galβ1-4GlcNAcβ1-3Galβ1-4Glc) and L2L2 are 0.28:0.72 (B3GNT1), 4.93:0.99 (B3GNT2), and 0.19:3.79 (B3GNT7). If you would use crude fractions for enzymatic source, it would be recommended to distinguish B3GNT7 activity from B3GNT1 and B3GNT2 ones that you also measure N-acetylglucosaminyltransferase activities for LNnT.
- Keratan sulfate and L2L2 are prepared by the method of Ref. 3. agL2L2 was prepared from L2L2 by digestion with Streptococcus 6646K β-galactosidase (Ref. 4). However, within my knowledge, at present, Streptococcus 6646K β-galactosidase, keratan sulfate, and L2L2 are not commercially available. Other β-galactosidases acting on β1-4-linkage might be substituted. Alternative acceptor substrate for B4GALT4 is GlcNAc 6-O-sulfate (Ref. 1). Similarly, L2L2 for B3GNT7 could be replaced with Galβ1-4(SO3--6)GlcNAc, which can be enzymatically synthesized from GlcNAc 6-O-sulfate by B4GALT4 (Ref. 1). Otherwise, bovine milk β1,4-galactosyltransferase, B4GALT1, may be applicable for the synthesis.
- Purification procedure of B4GALT4 from porcine colonic mucosa was reported in Ref. 1.
- In vitro biosynhesis of partial structure of keratan sulfate using with recombinant enzymes was reported in Ref. 5.
- There is danger of an electric shock when using high-voltage paper electrophoresis unit. Make sure of turning off the electric unit when papers would be set into the unit or taken out from it.
|
| Figure & Legends |
Figure & Legends

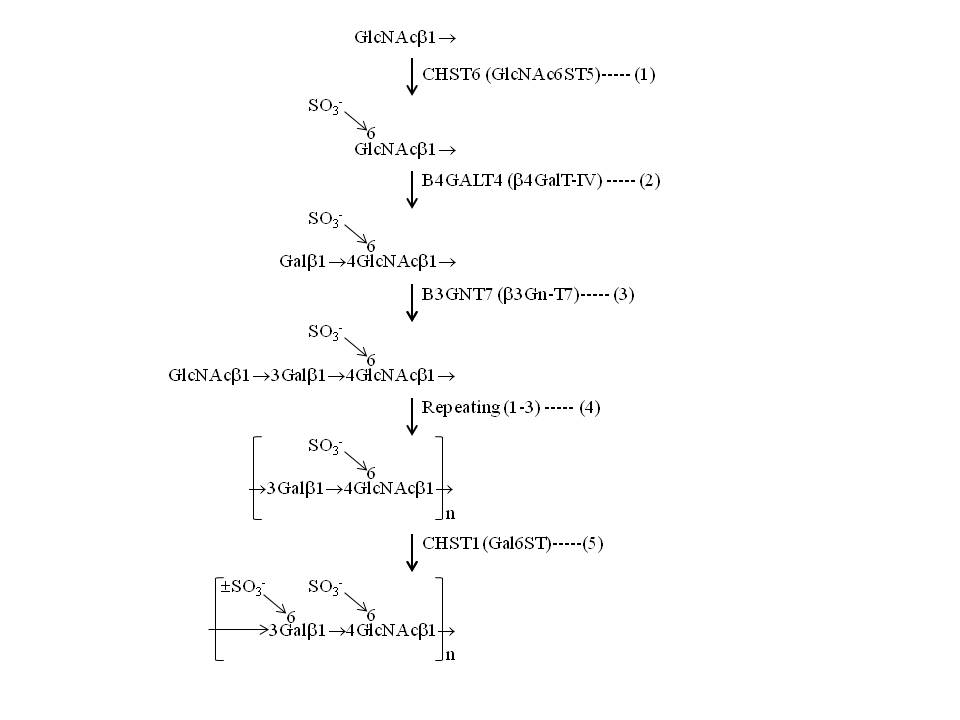
Fig. 1. Proposed scheme for the biosynthesis of keratan sulfate. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-30 09:17:46 |
- Seko, A., Dohmae, N., Takio, K., and Yamashita, K. (2003) β1,4-Galactosyltransferase (β4GalT)-IV is specific for GlcNAc 6-O-sulfate. β4GalT-IV acts on keratan sulfate-related glycans and a precursor glycan of 6-sulfosialyl-Lewis X. J. Biol. Chem. 278, 9150–9158 [PMID : 12511560]
- Seko, A., and Yamashita, K. (2004) β1,3-N-acetylglucosaminyltransferase-7 (β3Gn-T7) acts efficiently on keratan sulfate-related glycans. FEBS Lett. 556, 216–220 [PMID : 14706853]
- Brown, G.M., Huckerby, T.N., and Nieduszynski, I.A. (1994) Oligosaccharides derived by keratanase II digestion of bovine articular cartilage keratan sulphates. Eur. J. Biochem. 224, 281–308 [PMID : 7925342]
- Kiyohara, T., Terao, T., Shioiri-Nakano, K., and Osawa, T. (1976) Purification and characterization of β-N-acetylhexosaminidases and β-galactosidase from Streptococcus 6646 K. J. Biochem. 80, 9–17 [PMID : 9384]
- Kitayama, K., Hayashida, Y., Nishida, K., and Akama T.O. (2007) Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J. Biol. Chem. 282, 30085–30096 [PMID : 17690104]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Seko, Akira,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.25,4,2024 .
How to Cite this Work in Website:
Seko, Akira,
(2014).
Enzyme assay of GAG glycosyltransferases for keratan sulfate.
Retrieved 25,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t201.
html source
Seko, Akira,
(2014).
<b>Enzyme assay of GAG glycosyltransferases for keratan sulfate</b>.
Retrieved 4 25,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t201" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t201</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|