Chondroitin sulfates are generally degraded to oligosaccharides by chondroitinase. Bacterial chondroitinase is a lyase that cleaves N-acetyl-D-galactosaminidic linkages in an eliminative fashion to give 4,5-unsaturated uronic acid residue at the non-reducing end. The unsaturated uronic acid is an artificial form, while it is the saturated sugar residue at the non-reducing end in glycosaminoglycans. Thus, digestion with testicular hyaluronidase is preferred as a method of oligosaccharide collection from chondroitin sulfate as an intact form (Fig. 1). In this section, preparation of oligosaccharides from squid cartilage chondroitin sulfate E with testicular hyaluronidase is described. |
| Category | Glycosaminoglycans |
| Protocol Name | Chondroitin sulfate oligosaccharides |
Authors
 |
Kinoshita-Toyoda, Akiko
Laboratory of Bio-analytical Chemistry, College of Pharmaceutical Sciences, Ritsumeikan University
|
| KeyWords |
|
Reagents
 |
| ● |
Sheep testicular hyaluronidase (EC 3.2.1.35) (Sigma-Aldrich, St. Louis, MO) |
|
Instruments
 |
| ● |
Bio-Gel P-10 resin (Bio-Rad Laboratories, Hercules, CA) |
| ● |
Sephadex G-25 fine resin (GE Healthcare, Little Chalfont, UK) |
| ● |
Speed Vac Concentrator (Thermo Fisher Scientific Inc., Waltham, MA) |
|
| Methods |
|
1. |
Chondroitin sulfate oligosaccharides |
| 1) |
Incubate chondroitin sulfate E (100 mg) with hyaluronidase (10 mg, approximately 15,000 National formulary units) in a total volume of 2 mL of 50 mM sodium phosphate buffer, pH 6.0, containing 150 mM NaCl at 37˚C. |
Comment 1
|

|
| 2) |
Withdraw an aliquot of 2 μL of the sample at 1 h intervals to monitor the reaction. |
Comment 0
|

|
| 3) |
Mix the aliquot with H2O (1 mL) and boil at 100˚C for 2 min. |
Comment 0
|

|
| 4) |
Add 50 μL of 2.5% cetrimide containing 2% NaOH to an aliquot of 25 μL of the mixture and measure the absorbance at 400 nm. |
Comment 0
|

|
| 5) |
Add 4 mg (approximately 6,000 National formulary units) of the additional enzyme into the reaction mixture and incubate to complete the digestion. |
Comment 0
|

|
| 6) |
Add 0.44 mL of 30% trichloroacetic acid and centrifuge at 2500 rpm for 10 min. |
Comment 0
|

|
| 7) |
Wash the precipitate with 0.5 mL of 5% trichloroacetic acid. |
Comment 0
|

|
| 8) |
Extract the combined supernatant with 6 mL ether. |
Comment 0
|

|
| 9) |
Centrifuge at 1000 rpm for 3 min and remove the ether phase. |
Comment 0
|

|
| 11) |
Remove the ether phase completely. |
Comment 0
|

|
| 12) |
Neutralize the aqueous phase with 1 M Na2CO3. |
Comment 0
|

|
| 13) |
Concentrate the neutralized solution by Speed Vac to a final volume of less than 2 mL. |
Comment 0
|

|
| 14) |
Fractionate the sample by gel filtration chromatography on a Bio-Gel P-10 column (Fig. 2). |
Comment 1
|

|
| 15) |
Desalt the subfractions by gel filtration chromatography on a Sephadex G-25 fine column. |
Comment 1
|

|
| 16) |
Dry up the flow-through fraction by Speed Vac. |
Comment 0
|
|
|
| Figure & Legends |
Figure & Legends 
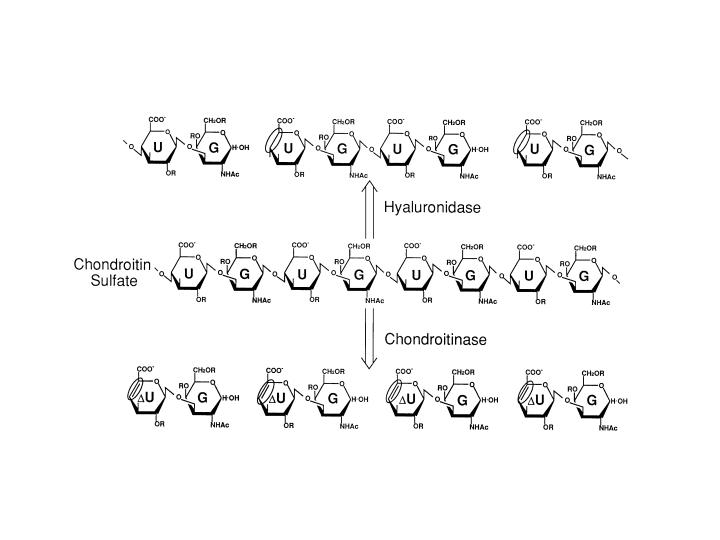
Fig. 1. Action of hyaruronidase and chondroitinase
Testicular hyaluronidase cleaves the N-acetyl-D-galactosaminidic linkages in CS chains in a hydrolytic fashion to yield tetra-, hexa-, octa-, deca-, and polysaccharides with glucuronic acid at the nonreducing ends as major products. In contrast, bacterial chondroitinase is a lyase that cleaves N-acetyl-D-galactosaminidic linkages in an eliminative fashion to give unsaturated disaccharides as major products. G: N-acetyl-D-galactosamine, U: glucuronic acid, ΔU: unsaturated uronic acid.

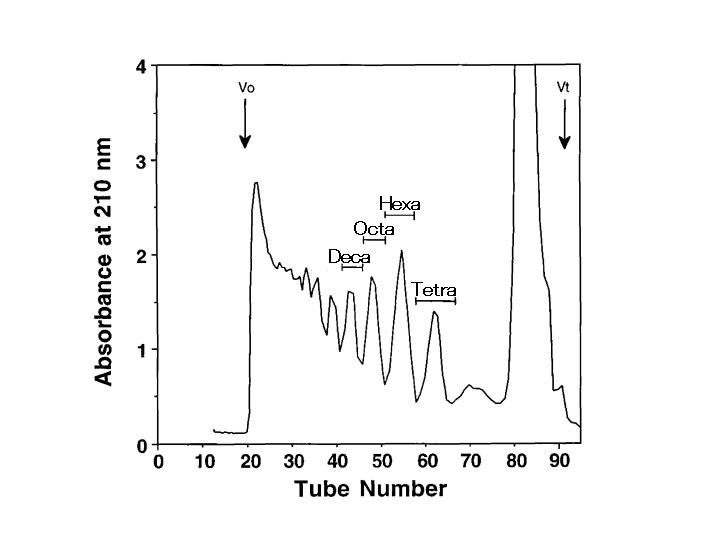
Fig. 2. Gel filtration column chromatography of the hyaluronidase digest of Squid cartilage chondroitin sulfate E on a Bio-Gel P-10 column
Squid cartilage chondroitin sulfate E was exhaustively digested with sheep testicular hyaluronidase. The digest was fractionated on a Bio-Gel P-10 column (1.6 cm i.d. x 95 cm) using 1 M NaCl, 10% ethanol as the eluent. Fractions (2 mL) were monitored by absorbance at 210 nm. The sizes of oligosaccharides are indicated.
This figure was originally published in J Biol Chem. Kinoshita A. et al. "Novel tetrasaccharides isolated from squid cartilage chondroitin sulfate E contain unusual sulfated disaccharide units GlcA(3-O-sulfate)beta1-3GalNAc(6-O-sulfate) or GlcA(3-O-sulfate)beta1-3GalNAc" 1997, 272(32):19656-65. © the American Society for Biochemistry and Molecular Biology. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-04-24 15:50:49 |
- Kinoshita A, Yamada S, Haslam SM, Morris HR, Dell A, Sugahara K. (1997) Novel tetrasaccharides isolated from squid cartilage chondroitin sulfate E contain unusual sulfated disaccharide units GlcA(3-O-sulfate)β1-3GalNAc(6-O-sulfate) or GlcA(3-O-sulfate) β1-3GalNAc(4,6-O-sulfate). J. Biol. Chem. 272: 19656-19665. [PMID : 9242620]
- Kinoshita A, Yamada S, Haslam SM, Morris HR, Dell A, Sugahara K. (2001) Isolation and structural determination of novel sulfated hexasaccharides from squid cartilage chondroitin sulfate E that exhibits neuroregulatory activities. Biochemistry 40: 12654-12665. [PMID : 11601990]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Kinoshita-Toyoda, Akiko,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.19,4,2024 .
How to Cite this Work in Website:
Kinoshita-Toyoda, Akiko,
(2014).
Chondroitin sulfate oligosaccharides.
Retrieved 19,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t19.
html source
Kinoshita-Toyoda, Akiko,
(2014).
<b>Chondroitin sulfate oligosaccharides</b>.
Retrieved 4 19,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t19" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t19</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|