Chondroitin sulfate (CS) and dermatan sulfate (DS) are ones of the major glycosaminoglycans, and covalently attached to various core proteins including aggrecan and versican (CS), and decorin and biglycan (DS). They are classified into several types according to their sugar composition and the position of sulfate modification (Fig. 1). Their sulfation is catalyzed by several linkage-specific sulfotransferases, which transfer sulfate residue from adenosine 3’-phosphate 5’-phosphosulfate (PAPS) to specific OH-groups of backbone glycan chains. So far, sulfotransferase genes responsible for the biosynthesis of CS/DS have been identified in human; chondroitin 4-O-sulfotransferases (C4ST-1, -2, and -3), dermatan 4-O-sulfotransferase (DS4ST), GalNAc-4-O-sulfate: 6-O-sulfotransferase (GalNAc4S-6ST), and uronosyl 2-O-sulfotransferase (2OST)(Fig. 2). Assays of the catalytic activities of these enzymes can be performed as shown in Fig. 3; incorporation of [35S]sulfate into glycosaminoglycan chains by sulfotransferase activities, and analysis of the linkage position of [35S]sulfate by specific enzyme digestion and following an anion-exchange HPLC. |
| Category | Glycosaminoglycans |
| Protocol Name | Assays of sulfotransferases involved in the biosynthesis of chondroitin sulfate and dermatan sulfate |
Authors
 |
Seko, Akira
Ito Glycotrilogy Project, Japan Science and Technology Agency (JST)
|
| KeyWords |
|
Reagents
 |
| ● |
Adenosine 3’-phosphate 5’-phospho[35S]sulfate (PerkinElmer, Waltham, MA) |
| ● |
Chondroitin sulfate A (CS-A) from bovine trachea (Sigma-Aldrich, St. Louis, MO) |
| ● |
Dermatan sulfate (CS-B or DS) from porcine intestine (Sigma-Aldrich) |
| ● |
Chondroitin sulfate C (CS-C) from shark cartilage (Sigma-Aldrich) |
| ● |
Partially desulfated CS and DS
Partially desulfated CS and DS are prepared by the methods of Nagasawa et al., (1979). The pyridinium salt of CS or DS is dissolved in DMSO/methanol (9:1, v/v) and incubated at 80°C for 2 h.
|
| ● |
Chondroitinase ABC from Proteus vulgaris (Sigma-Aldrich) |
| ● |
ΔDi-0S, 2-acetamide-2-deoxy-3-O-(β-D-gluco-4-enepyranosyluronic acid)-D-galactose (Dextra Laboratories Ltd., Reading, UK or Sigma-Aldrich) |
| ● |
ΔDi-4S, 2-acetamide-2-deoxy-3-O-(β-D-gluco-4-enepyranosyluronic acid)-4-O-sulfo-D-galactose (Dextra Laboratories Ltd. or Sigma-Aldrich) |
| ● |
ΔDi-6S, 2-acetamide-2-deoxy-3-O-(β-D-gluco-4-enepyranosyluronic acid)-6-O-sulfo-D-galactose (Dextra Laboratories Ltd. or Sigma-Aldrich) |
| ● |
ΔDi-diSD, 2-acetamide-2-deoxy-3-O-(2-O-sulfo-β-D-gluco-4-enepyranosyluronic acid)-6-O-sulfo-D-galactose (Dextra Laboratories Ltd.) |
| ● |
ΔDi-diSB, 2-acetamide-2-deoxy-3-O-(2-O-sulfo-β-D-gluco-4-enepyranosyluronic acid)-4-O-sulfo-D-galactose (Dextra Laboratories Ltd.) |
| ● |
ΔDi-diSE, 2-acetamide-2-deoxy-3-O-(β-D-gluco-4-enepyranosyluronic acid)-4,6-bis-O-sulfo-D-galactose (Dextra Laboratories Ltd.) |
|
Instruments
 |
| ● |
High-voltage paper electrophoresis system (Advantec Co., Ltd., Ehime, Japan) |
| ● |
Radiochromatogram scanner (Raytest, Straubehardt, Germany) |
| ● |
|
| ● |
Partisil-10 SAX column (4.6 × 250 mm, GE Healthcare, Little Chalfont, UK) |
| ● |
Superdex 30 16/60 column (1.6 × 60 cm, GE Healthcare) |
|
| Methods |
|
1. |
Enzymatic reactions of chondroitin 4-O-sulfotransferase and dermatan 4-O-sulfotransferase (Mikami et al., 2003) |
| 1) |
Fifty μL of the reaction mixtures containing 50 mM imidazole-HCl (pH 6.8), 2 mM DTT, 10 μM [35S]PAPS (1.1 × 106 dpm), acceptor glycosaminoglycans (partially desulfated CS and partially desulfated DS, respectively; 10 nmol as GlcA), and enzyme fraction, are incubated at 37°C for 1 h. |
Comment 0
|

|
| 2) |
The reaction is stopped by heating at 100°C for 3 min. |
Comment 0
|
|
|
|
2. |
Enzymatic reaction of chondroitin 6-O-sulfotransferase (Habuchi et al., 1993) |
| 1) |
Fifty μL of the reaction mixtures containing 50 mM imidazole-HCl (pH 6.8), 2 mM DTT, 10 μM [35S]PAPS (1.1 × 106 dpm), acceptor partially desulfated CS (25 nmol as GlcA), 1.25 μg protamine-HCl, and enzyme fraction, are incubated at 37°C for 20 min. |
Comment 0
|

|
| 2) |
The reaction is stopped by heating at 100°C for 3 min. |
Comment 0
|
|
|
|
3. |
Enzymatic reaction of uronosyl 2-O-sulfotransferase (Ohtake et al., 2005) |
| 1) |
Fifty μL of the reaction mixtures containing 50 mM imidazole-HCl (pH 6.8), 10 μM [35S]PAPS (1.1 × 106 dpm), acceptor CS-A (25 nmol as galactosamine), 2.6 μg protamine-HCl, and enzyme fraction, are incubated at 37°C for 30 min. |
Comment 0
|

|
| 2) |
The reaction is stopped by heating at 100°C for 3 min. |
Comment 0
|
|
|
|
4. |
Enzymatic reaction of GalNAc-4-O-sulfate: 6-O-sulfotransferase (Ito and Habuchi, 2000) |
| 1) |
Fifty μL of the reaction mixtures containing 50 mM imidazole-HCl (pH 6.8), 20 mM CaCl2, 10 μM [35S]PAPS (1.1 × 106 dpm), acceptor CS-A (25 nmol as galactosamine), 20 mM reduced glutathione, and enzyme fraction, are incubated at 25°C for 20 min. |
Comment 0
|

|
| 2) |
The reaction is stopped by heating at 100°C for 3 min. |
Comment 0
|
|
|
|
5. |
Analysis of the reaction products |
| 1) |
The reaction mixtures are applied to paper electrophoresis (pyridine/acetic acid/water, 3:1:387, pH 5.4). The paper is dried and the radioactivity is monitored by a radiochromatogram scanner. The Rf value of PAPS is approximately 1.9, when the Rf value of bromophenol blue is taken as 1.0. The enzymatic products, 35S-labeled glycosaminoglycans, move broadly from origin approximately to the position of bromophenol blue. |
Comment 0
|

|
| 2) |
The products are extracted from the paper with water and dried. |
Comment 0
|

|
| 3) |
The purified products are analyzed for the disaccharide compositions according to Ohtake et al., (2005). The 35S-labeled products are digested with chondroitinase ABC at 37°C for 24 h [50 mM Tris-HCl (pH 8.0), 50 mM sodium acetate, 0.1 mg/mL BSA, and 30 mU of the enzyme]. |
Comment 0
|

|
| 4) |
The digests are applied to Superdex 30 gel filtration (equilibrated and eluted with 0.2 M NH4HCO3) to separate radiolabeled disaccharides from tetra-saccharides, hexa-saccharides, and larger saccharides. The disaccharide fractions are collected and dried. |
Comment 0
|

|
| 5) |
The disaccharide fractions are applied on anion-exchange Partisil-10 SAX column (equilibrated with 5 mM KH2PO4, and eluted with a linear gradient from 5 to 500 mM KH2PO4). The fractions are collected, and counted for the radioactivity. The elution positions of authentic sulfated disaccharides are determined by monitoring the absorbance at 232 nm. |
Comment 0
|
|
|
| Discussion | To determine the linkage position of incorporated [35S]sulfate, it is necessary to perform digestion of 35S-labeled disaccharides with linkage-specific sulfatases. So far, chondro-4-sulfatase and chondro-6-sulfatase from Proteus vulgaris, and 2-O-sulfatase from Flavobacterium heparinum, were extensively studied for their substrate specificities (Sugahara and Kojima, 1996). However, within my knowledge, these enzymes are not commercially available now. I can only note that the purification procedure of the former two from the culture of Proteus vulgaris was shown by Yamagata et al. (1968), and that the purification procedure of the 2-O-sulfatase and its molecular cloning were shown by McLean et al., (1984) and Myette et al., (2003), respectively. |
| Figure & Legends |
Figure & Legends

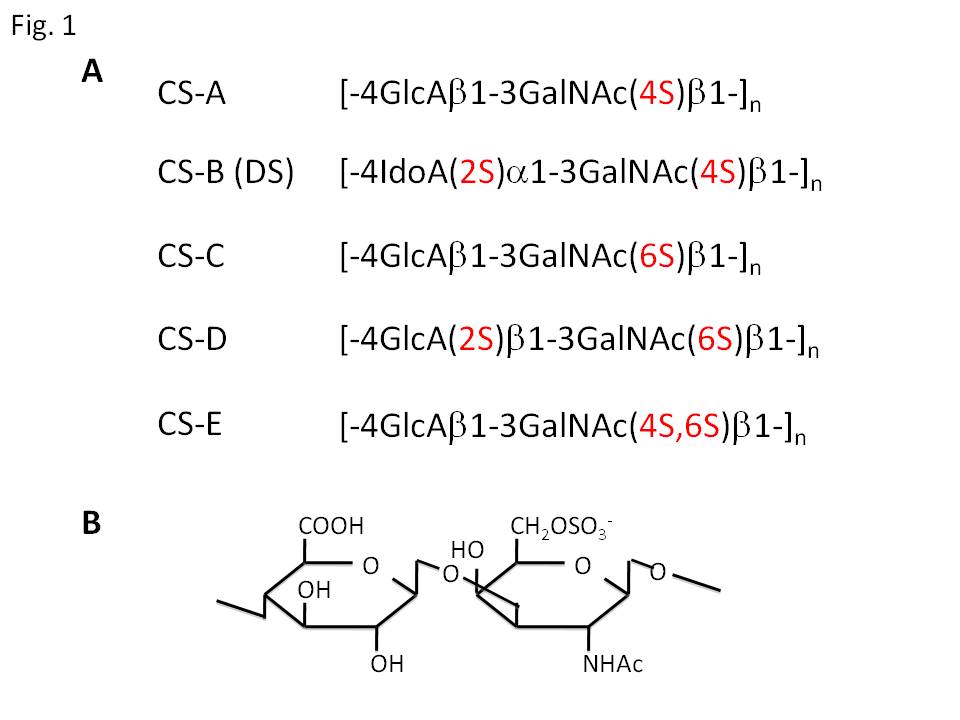
Fig. 1. (A) Major structures of repeating disaccharide units of chondroitin sulfate (CS) and dermatan sulfate (DS). CS is composed of repeating disaccharide unit (GlcAβ1-3GalNAcβ1-4) with modification of sulfate at subtype-specific OH-groups. Present disaccharide structures are major ones of respective subtype, and generally CS and DS chains contain microheterogeneity for sulfate positions. GlcA, D-glucuronic acid; GalNAc, N-acetyl-D-galactosamine; IdoA, L-iduronic acid. (B) Structure of the major disaccharide unit of CS-C.

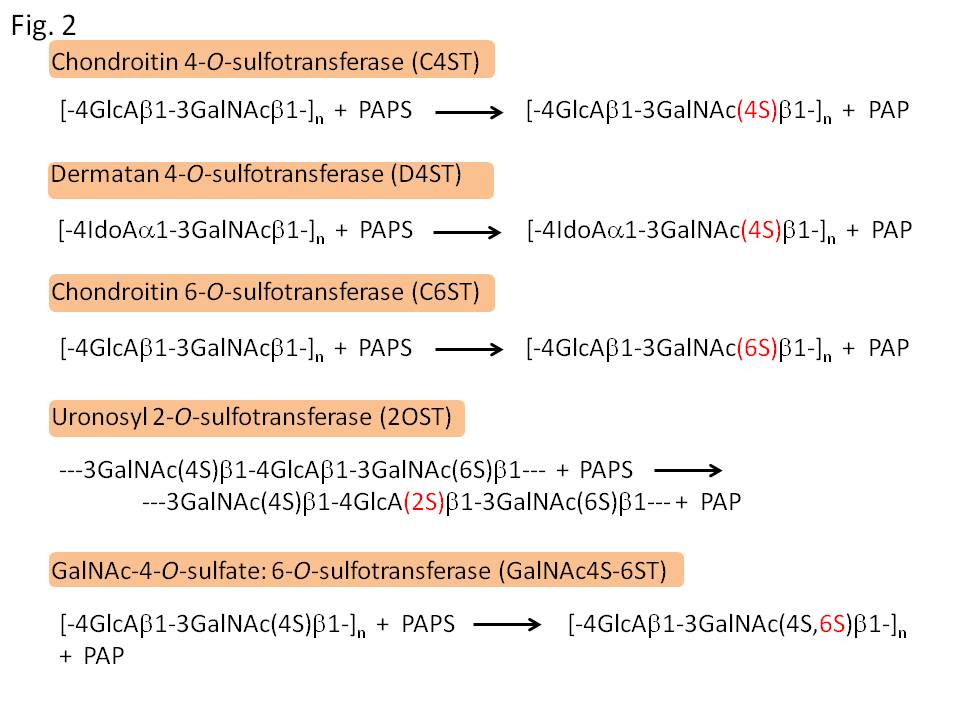
Fig. 2. Catalytic reactions of sulfotransferases for the biosynthesis of CS and DS. C4ST, D4ST, and C6ST initially act on backbone glycan chains, and thereafter 2OST and GalNAc4S-6ST work to synthesize more complex units. PAPS, adenosine 3’-phosphate 5’-phosphosulfate; PAP, adenosine 3’5’-diphosphate.

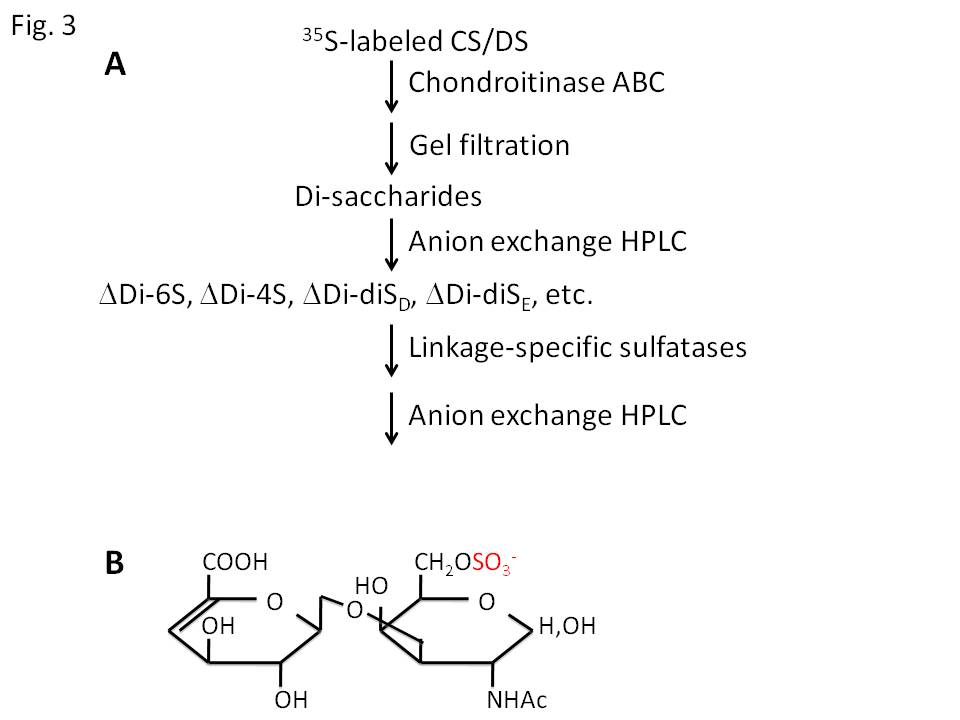
Fig. 3. (A) Scheme of assay of CS/DS sulfotransferase activities. 35S-labeled CS/DS produced by the enzymatic reactions are purified and digested with chondroitinase ABC. This enzyme is an eliminase, and cleaves the reducing side of GalNAc residues to produce sulfated Δ4-hexuronic acid-GalNAc disaccharides. Sulfated disaccharides are separated by anion exchange HPLC and the linkage positions of sulfate are determined in comparison with authentic disaccharides. (B) Structure of ΔDi-6S. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-30 09:17:12 |
- Mikami, T., Mizumoto, S., Kago, N., Kitagawa, H., and Sugahara, K. (2003) Specificities of three distinct human chondroitin/dermatan N-acetylgalactosamine 4-O-sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor. Implication of differential roles in dermatan sulfate biosynthesis. J. Biol. Chem. 278, 36115–36127 [PMID : 12847091]
- Habuchi, O., Matsui, Y., Kotoya, Y., Aoyama, Y., Yasuda, Y., and Noda, M. (1993) Purification of chondroitin 6-sulfotransferase secreted from cultured chick embryo chondrocytes. J. Biol. Chem. 268, 21968–21974 [PMID : 8408053]
- Ohtake, S., Kimata, K., and Habuchi, O. (2005) Recognition of sulfation pattern of chondroitin sulfate by uronosyl 2-O-sulfotransferase. J. Biol. Chem. 280, 39115–39123 [PMID : 16192264]
- Ito, Y., and Habuchi, O. (2000) Purification and characterization of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase from the squid cartilage. J. Biol. Chem. 275, 34728–34736 [PMID : 10871629]
- Nagasawa, K., Inoue, Y., and Tokuyasu, T. (1979) An improved method for the preparation of chondroitin by solvolytic desulfation of chondroitin sulfates. J. Biochem. (Tokyo) 86, 1323–1329 [PMID : 521436]
- Sugahara, K., and Kojima, T. (1996) Specificity studies of bacterial sulfatases by means of structurally defined sulfated oligosaccharides isolated from shark cartilage chondroitin sulfate D. Eur. J. Biochem. 239, 865–870 [PMID : 8774737]
- Yamagata, T., Saito, H., Habuchi, O., and Suzuki, S. (1968) Purification and properties of bacterial chondroitinases and chondrosulfatases. J. Biol. Chem. 243, 1523–1535 [PMID : 5647268]
- McLean, M.W., Bruce, J.S., Long, W.F., and Williamson, F.B. (1984) Flavobacterium heparinum 2-O-sulphatase for 2-O-sulphato-delta 4,5-glycuronate-terminated oligosaccharides from heparin. Eur. J. Biochem. 145, 607–615 [PMID : 6510419]
- Myette, J.R., Shriver, Z., Claycamp, C., McLean, M.W., Venkataraman, G., and Sasisekharan, R. (2003) The heparin/heparin sulfate 2-O-sulfatase from Flavobacterium heparinum. Molecular cloning, recombinant expression, and biochemical characterization. J. Biol. Chem. 278, 12157–12166 [PMID : 12519775]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Seko, Akira,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.20,4,2024 .
How to Cite this Work in Website:
Seko, Akira,
(2014).
Assays of sulfotransferases involved in the biosynthesis of chondroitin sulfate and dermatan sulfate.
Retrieved 20,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t169.
html source
Seko, Akira,
(2014).
<b>Assays of sulfotransferases involved in the biosynthesis of chondroitin sulfate and dermatan sulfate</b>.
Retrieved 4 20,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t169" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t169</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|