Immunofluorescence staining method is widely used and a powerful technique to detect localization of proteins on cells and tissues with high sensitivity. This unit describes the direct staining of specific carbohydrate structures with a fluoresceinated lectin. The procedure relies on proper fixation of cells to retain cellular distribution of antigen and to preserve cellular morphology. |
| Category | Sugar binding proteins |
| Protocol Name | Histochemical analysis of cell surface glycans by fluoresceinated lectin |
Authors
 |
Nonaka, Motohiro
Laboratory for Drug Discovery, Biotechnology Research Institute for Drug Discovery, Department of Life Science and Biotechnology, National Institute of Advanced Industrial Science and Technology (AIST)
|
Reagents
 |
| ● |
FITC (Fluorescein isothiocyanate) |
| ● |
|
| ● |
|
| ● |
50mM borate buffer, pH 8.5 |
| ● |
Phosphate-buffered saline (PBS), pH 7.4 |
| ● |
|
| ● |
|
| ● |
|
| ● |
Acetone or 4% paraformaldehyde (PFA) in PBS, pH 7.4 |
| ● |
Blocking solution (1% BSA in PBS) |
| ● |
|
| ● |
|
|
Instruments
 |
|
| Methods |
|
1. |
Direct labeling of lectin with FITC |
| 1) |
Dissolve FITC in DMF at 10 mg/mL. |
Comment 0
|

|
| 2) |
Mix well to completely dissolve the FITC. |
Comment 0
|

|
| 3) |
Dissolve the lectin up to 1 mg in 0.5 mL of 50 mM borate buffer, pH 8.5. |
Comment 1
|

|
| 4) |
Add 15 to 20-fold molar excess of FITC to 0.5 mL of lectin solution and immediately mix it. |
Comment 0
|

|
| 5) |
Incubate for 1 h at room temperature in the dark. |
Comment 0
|

|
| 6) |
Remove excess and hydrolyzed FITC by dialysis. |
Comment 0
|

|
| 7) |
Store the fluoresceinated lectin at 4°C in the dark. |
Comment 0
|
|
|
|
2. |
Freezing the tissue sample |
| 1) |
Place the freshly isolated tissue in a petri dish containing PBS and precut piece of aluminum foil. |
Comment 0
|

|
| 2) |
Apply OCT embedding compound to the fragment sufficiently to completely cover the tissue. |
Comment 0
|

|
| 3) |
Fold the foil to create a secure envelope. |
Comment 0
|

|
| 4) |
Drop the foil envelope directly into liquid nitrogen. |
Comment 0
|

|
| 5) |
Incubate the specimen for 5 to 10 min. |
Comment 1
|
|
|
|
3. |
Cryosection of the tissue |
| 1) |
Open the aluminum foil envelope containing the tissue inside the cryostat. |
Comment 0
|

|
| 2) |
Place the tissue on the OCT-coated chuck in the appropriate orientation. |
Comment 1
|

|
| 3) |
Allow the tissue to freeze solid for 10 min at −20°C inside the cryostat. |
Comment 0
|

|
| 4) |
Set the cryostat to cut 3 to 8-μm-thick sections. |
Comment 0
|

|
| 5) |
Allow the glass slide with the cryostat sections to air dry for more than 30 min. |
Comment 0
|
|
|
|
4. |
|
| 1) |
Immerse the slides 5 min in a Coplin jar containing acetone or 4% PFA-PBS. |
Comment 1
|

|
| 2) |
Allow the slides to air dry 10 min at room temperature. |
Comment 0
|
|
|
|
5. |
|
| 1) |
Using a pen containing water-repellant wax, outline the tissue sections on the glass slide. |
Comment 0
|

|
| 2) |
Place the slide in a staining chamber. |
Comment 0
|

|
| 3) |
Add blocking solution to the slides. |
Comment 0
|

|
| 5) |
Remove as much of the solution as possible by tilting the slides. |
Comment 0
|

|
| 6) |
Apply fluoresceinated lectin in sufficient quantity to cover the tissue. |
Comment 1
|

|
| 7) |
Incubate at room temperature for 1 h in the dark. |
Comment 0
|

|
| 8) |
Wash the slides three times for 5 min each. |
Comment 0
|
|
|
|
6. |
|
| 1) |
Place 1 drop of mounting medium onto slide. |
Comment 0
|

|
| 3) |
Gently blot mounted coverslip with paper towel. |
Comment 0
|

|
| 4) |
Seal edge of coverslip onto slide by painting the edge with a rim of nail polish and let dry. |
Comment 0
|

|
| 5) |
View specimen on fluorescence microscope. |
Comment 1
|
|
|
| Notes | If background staining with lectin is too high, the lectin solution may be diluted further, wash for longer time, or try higher concentration of BSA in blocking solution. If specific staining is observed, but it is very faint, it is possible to increase the concentration of the lectin solution. Tissues can be counterstained with DAPI if necessary. |
| Figure & Legends |
Figure & Legends 
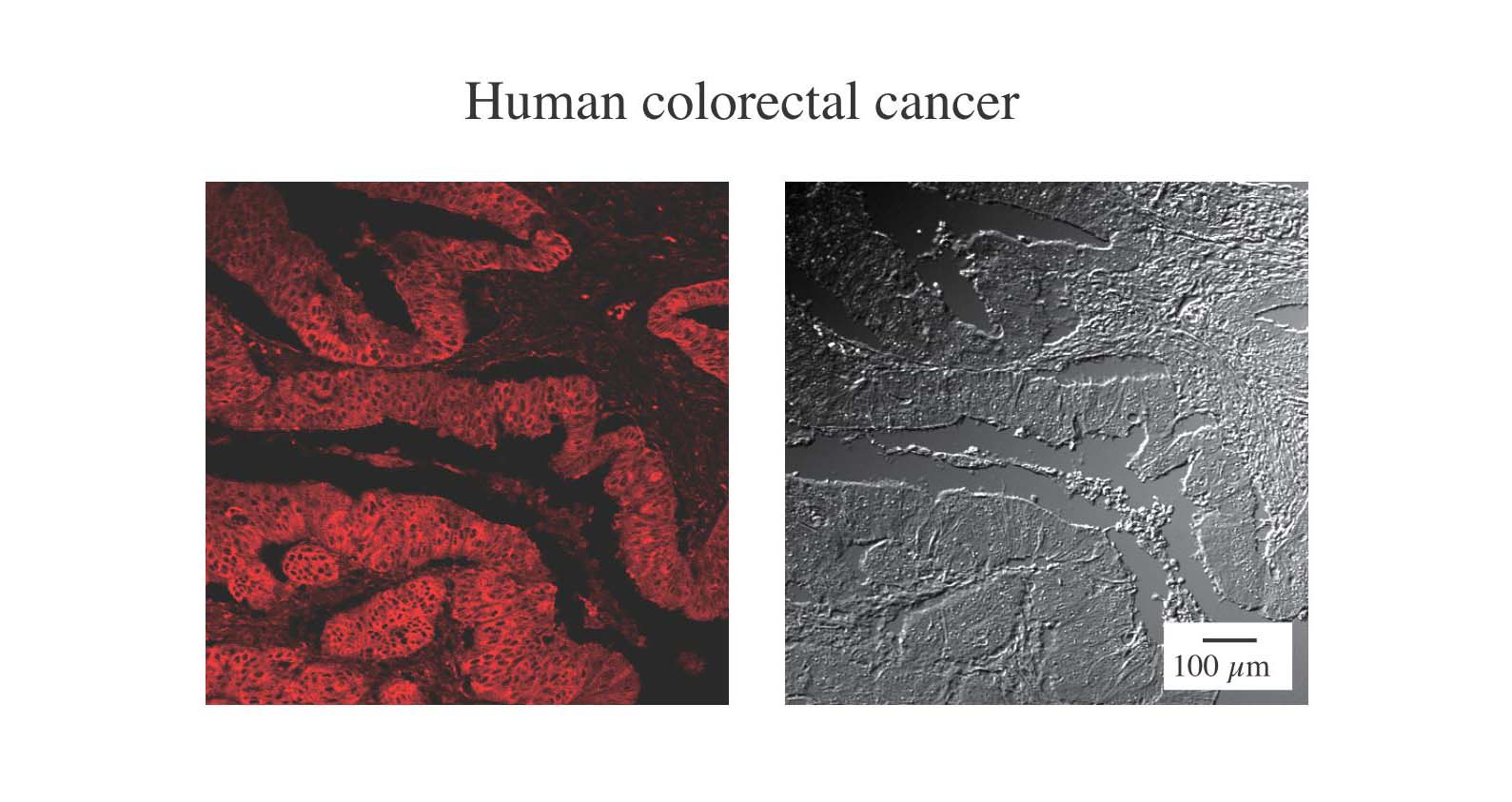
Fig. 1. Histochemical analysis of colorectal cancer-derived tissue stained by APC-conjugated DC-SIGN
The section of human malignant colon tissue was stained with APC-conjugated animal lectin DC-SIGN. The expressions of DC-SIGN ligands were visualized by laser confocal microscopy. The Nomarski images are shown in the right panel. This indicates DC-SIGN ligands are highly expressed on colon cancer epithelia. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2015-02-06 11:13:00 |
- Current protocols in immunology (Vol. 5), John Wiley & Sons, Inc.
- Shin-sensyokuhou (Medical Technology Sup.), Ishiyaku Publishers, Inc.
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Nonaka, Motohiro,
(2015). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.27,4,2024 .
How to Cite this Work in Website:
Nonaka, Motohiro,
(2015).
Histochemical analysis of cell surface glycans by fluoresceinated lectin.
Retrieved 27,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t16.
html source
Nonaka, Motohiro,
(2015).
<b>Histochemical analysis of cell surface glycans by fluoresceinated lectin</b>.
Retrieved 4 27,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t16" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t16</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|