Lymphocyte homing is mediated by adhesive interactions between L-selectin on lymphocytes and its carbohydrate ligands on high endothelial venules (HEVs) in peripheral lymph nodes (PLNs). Originally, Stamper and Woodruff developed an in vitro adhesion assay to detect selective binding of lymphocytes to HEVs on tissue sections, which has significantly contributed for the identification of molecules involved in lymphocyte homing. Herein, I will describe a modified version of the Stamper-Woodruff cell-binding assay using lymphocytes labeled with a fluorescent orange dye, CMTMR. In this method, lymphocytes bound to HEVs can be clearly visualized by fluorescence microscopy. |
| Category | Sugar binding proteins |
| Protocol Name | Modified Stamper-Woodruff cell-binding assay |
Authors
 |
Kawashima, Hiroto
Department of Biochemistry, Hoshi University School of Pharmacy and Pharmaceutical Sciences
|
| KeyWords |
|
Reagents
 |
| ● |
Mouse (10-week-old female C57BL/6 mouse) |
| ● |
OCT compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) |
| ● |
Aminosilane (APS)-coated glass slide (Matsunami Glass Ind., Ltd., Osaka, Japan) |
| ● |
Wax pencil (PAP PEN: Daido Sangyo Co., Ltd., Tokyo, Japan) |
| ● |
Bovine serum albumin (BSA: Sigma-Aldrich, St. Louis, MO) |
| ● |
Nylon mesh (100 μm, Nippon Rikagaku Kikai Co., Ltd., Tokyo, Japan) |
| ● |
HEPES (2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid: Dojindo Laboratories, Kumamoto, Japan) |
| ● |
Buffer A (20 mM HEPES-NaOH, 0.15 M NaCl, 1 mM CaCl2, 1 mM MgCl2 pH7.4) |
| ● |
Monoclonal antibody (mAb) S2 (anti-sulfated glycan mAb, mouse IgM, see ref. 2) |
| ● |
RPMI1640 (Invitrogen/Life Technologies, Carlsbad, CA) |
| ● |
FBS (fetal bovine serum heat-inactivated for 30 min at 56°C) |
| ● |
ACK solution (150 mM NH4Cl, 1 mM KHCO3, 0.1mM EDTA 2Na) |
| ● |
CellTrackerTM Orange CMTMR
(5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine: Lonza Walkersville, Inc., Walkersville, MD) |
| ● |
mAb MEL-14 (anti-mouse L-selectin mAb, rat IgG) |
| ● |
Glutaraldehyde solution 50% (Kanto Chemical Co., Inc.) |
| ● |
FluorMount (Diagnostic Biosystems Inc., Pleasanton, CA) |
|
Instruments
 |
| ● |
Shaker platform (Double Shaker NR-3: TAITEC Co., Ltd., Koshigaya, Japan) |
| ● |
Fluorescence microscope (BX51: Olympus, Tokyo Japan) |
|
| Methods |
|
1. |
Preparation of cryosections |
| 1) |
Embed mouse PLNs in OCT compound, place them on dry-ice until they are frozen, and store them at −80°C until sectioning. |
Comment 0
|

|
| 2) |
Prepare cryostat sections (7 μm) and mount them on APS-coated glass slides (4–5 PLNs per slide). |
Comment 0
|

|
| 3) |
Air-dry the sections for 10 min and fix them for 10 min in 0.5% glutaraldehyde in PBS. |
Comment 1
|

|
| 4) |
Dip the sections in tap water for 5 min. |
Comment 0
|

|
| 6) |
Remove excess fluid by gently tapping the glass slides against tissue paper and carefully wiping around the tissue sections. |
Comment 0
|

|
| 7) |
Circle the tissue sections with a wax pencil. |
Comment 0
|

|
| 8) |
Apply 300 μL of 3% BSA in buffer A to block non-specific binding sites for 45 min. |
Comment 0
|

|
| 9) |
Incubate sections with or without 10 μg/mL mAb S2 or control mouse IgM in 0.1% BSA in buffer A for at least 30 min. Keep the slides in a moisture chamber at 4°C until use. |
Comment 1
|
|
|
|
2. |
Preparation of lymphocyte suspension |
| 1) |
Mince mouse spleens (3 spleens) with a scalpel blade and gently press the tissues between glass slides to squeeze out lymphocytes in 5 mL RPMI1640 containing 10% FBS. |
Comment 1
|

|
| 2) |
Settle the cell suspension in a 15 mL tube for 5 min to remove debris. |
Comment 0
|

|
| 3) |
Pass the supernatant through a piece of nylon mesh (100 μm) and transfer the pass-through fraction (containing lymphocytes) into a 15 mL conical polypropylene tube. |
Comment 0
|

|
| 4) |
Centrifuge at 1,500 rpm (420 g) for 5 min at 4°C. |
Comment 0
|

|
| 5) |
Remove supernatant. Add 1 mL of ACK solution and incubate for 5 min at RT. |
Comment 0
|

|
| 6) |
Add 3 mL of RPMI1640 containing 10% FBS and centrifuge at 1,500 rpm (420 g) for 5 min at 4°C. |
Comment 0
|

|
| 8) |
Incubate the cells with 2 mL of 3.5 μM CellTracker Orange CMTMR (5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine; Lonza Walkersville, Inc.) in RPMI1640 without FBS for 10 min at 37°C. |
Comment 0
|

|
| 9) |
Add 8 mL of 0.1% BSA in buffer A and centrifuge at 1,500 rpm (420 g) for 5 min at 4°C. |
Comment 0
|

|
| 10) |
Remove supernatant. Add 10 mL of 0.1% BSA in buffer A and count the cell number. |
Comment 0
|

|
| 11) |
Centrifuge at 1,500 rpm (420 g) for 5 min at 4 °C. |
Comment 0
|

|
| 12) |
Remove the supernatant and incubate the cells in 0.1% BSA in buffer A at a cell density of 1 × 107 cells/mL in the presence or absence of 10 μg/mL MEL-14, control rat IgG, S2, or control mouse IgM in 0.1% BSA in buffer A for at least 10 min on ice before applying them to the glass slides in the Method 3, Adhesion assay. |
Comment 1
|
|
|
|
3. |
|
| 1) |
Remove fluid from the glass slides prepared in the Method 1 by gently tapping against tissue paper. |
Comment 1
|

|
| 2) |
Place the glass slides horizontally on a shaker platform operating at 60 rpm at 4°C in a cold room. |
Comment 0
|

|
| 3) |
Apply 100 μL (1 × 106 cells/section) of lymphocyte suspension (prepared in the Method 2 ) onto each glass slide while the shaker platform is operating. Continue the agitation for 30 min at 60 rpm. |
Comment 0
|

|
| 4) |
Take off the slides one by one from the shaker platform, remove excess fluid by gently tapping them against tissue paper, dip them gently in buffer A in a 50 mL centrifuge tube to remove non-adherent lymphocytes for 10 sec, and place them gently in a 50 mL centrifuge tube containing 0.5% glutaraldehyde in buffer A for 10 min. Have the shaker still working until the last slide is removed. |
Comment 1
|

|
| 5) |
Gently dip the sections twice in tap water. |
Comment 1
|

|
| 6) |
Mount the sections with FluorMount. |
Comment 0
|

|
| 7) |
After three washes with distilled water, observe lymphocyte adhesion by a fluorescence microscope (20× objective). |
Comment 0
|
|
|
| Notes | HEVs are present throughout the PLN cortex, predominantly in the paracortical area. HEVs can be identified by the plump columner appearance of their endothelial cells. Typically, lymphocytes specifically bind to HEVs in the assay described above. Various molecules including mAbs against various glycans or low molecular weight compounds can be tested for their ability to block L-selectin-dependent adhesion of lymphocytes to HEVs by this assay. |
| Discussion | As shown in Fig. 1, the binding of CMTMR-labeled lymphocytes to HEVs was completely inhibited by an anti-L-selectin mAb, MEL-14. Anti-carbohydrate mAb S2, which recognizes sulfated glycans on HEVs, also significantly blocked the binding of lymphocytes to HEVs. These results indicate that the binding of lymphocytes to HEVs is mainly mediated by L-selectin and sulfated glycans expressed on HEVs. |
| Figure & Legends |
Figure & Legends 
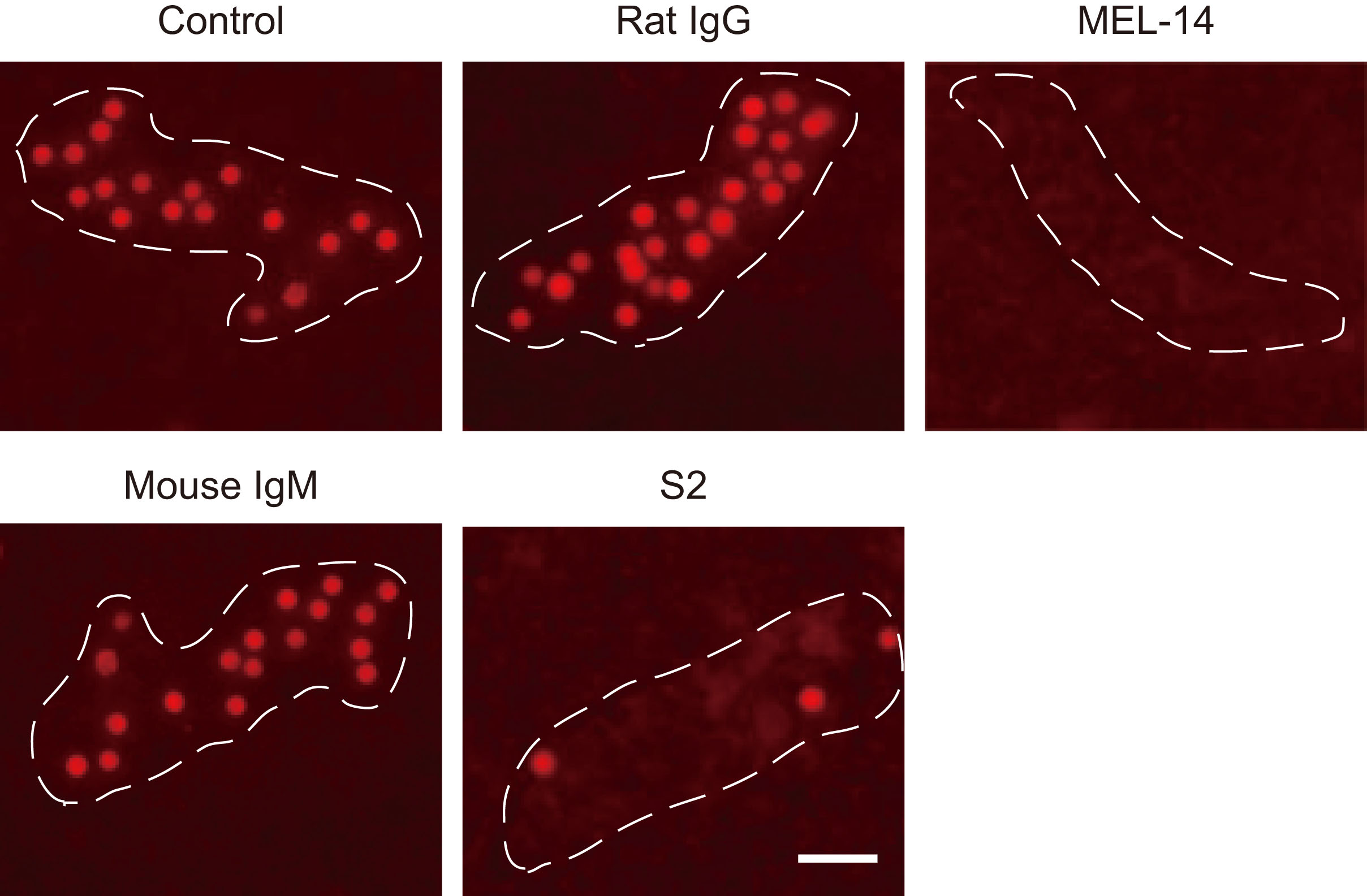
Fig. 1. Photomicrographs of lymphocyte binding to HEVs.
Binding of CMTMR-labeled lymphocytes to HEVs in the presence or absence (Control) of 10 μg/mL rat IgG, MEL-14, mouse IgM, or S2. Dotted line, outline of HEV. Bar, 40 μm.
This figure was originally published in J Biol Chem. Hirakawa J. et al. “Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing” 2010, 285:40864–40878. © the American Society for Biochemistry and Molecular Biology. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-12-11 14:35:26 |
- Stamper, H., B. Jr., and Woodruff, J. J. (1976) Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J. Exp. Med. 144, 828–833 [PMID : 956727]
- Hirakawa, J., Tsuboi, K., Sato, K., Kobayashi, M., Watanabe, S., Takakura, A., Imai, Y., Ito, Y., Fukuda, M., and Kawashima, H. (2010) Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J. Biol. Chem. 285, 40864–40878 [PMID : 20929857]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Kawashima, Hiroto,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.23,4,2024 .
How to Cite this Work in Website:
Kawashima, Hiroto,
(2014).
Modified Stamper-Woodruff cell-binding assay.
Retrieved 23,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t153.
html source
Kawashima, Hiroto,
(2014).
<b>Modified Stamper-Woodruff cell-binding assay</b>.
Retrieved 4 23,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t153" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t153</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|