Saccharide primers are amphiphilic glycosides having saccharide(s) and hydrocarbon chain to synthesize oligosaccharides using glycan biosynthetic pathway in cells. By incorporating the saccharide primers into cell culture medium, the saccharide primers are glycosylated in cells, and the glycosylated products are secreted in the culture medium. The sequences of the products are dependent on the glycan biosynthetic pathway in the cells. By combinating the saccharide primers and cell lines, it is possible to conveniently synthesize many kinds of oligosaccharides. Several saccharide primers such as Lac-C12, GlcNAc-C12, and GalNAc-Thr-C12 have been developed so far. Oligosaccharides such as ganglio-, globo-, lacto- or neolacto-series were obtained using Lac-C12, lacto- or neolacto-series oligosaccharides were obtained using GlcNAc-C12, and mucin-type oligosaccharides were obtained using GalNAc-Thr-C12. |
| Category | Large scale preparation of glycans, glycoproteins & glycolipids |
| Protocol Name | Cellular synthesis of oligosaccharides using saccharide primers |
Authors
 |
Sato, Toshinori
Department of Biosciences and Informatics, Keio University
|
| KeyWords |
|
Reagents
 |
| ● |
Dodecyl 2-acetamide-2-deoxy-β-D-glucopyranoside (GlcNAc-C12) |
| ● |
Dodecyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside (Lac-C12) |
| ● |
Na-Lauryl-O-(2-acetamido-2-deoxy-a-D-galactopyranosyl)-L-threonine-amido (GalNAc-Thr-C12) |
| ● |
Sep-Pak C18 column (Waters Corp., Milford, MA) |
| ● |
Amino column (Supelco/Sigma-Aldrich, Bellefonte, PA) |
| ● |
Glass fiber filter (ATTO Corporation, Tokyo, Japan) |
| ● |
PVDF membrane (ATTO Corporation) |
| ● |
PTFE membrane (ATTO Corporation) |
| ● |
Iatrobead column (6RSP-8005, 4.6 × 250 mm, Iatron Laboratories Inc., Tokyo, Japan) |
| ● |
HPTLC (Silica gel 60, Merck Millipore, Billerica, MA) |
| ● |
Resorcinol reagent (dissolve 200 mg resorcinol (Nacalai Tesque Inc., Kyoto, Japan) in 10 mL H2O, 80 mL HCl, 0.25 mL 0.1 M CuSO4 aq. solution , and dilute with H2O to 100 mL) |
| ● |
Orcinol reagent (dissolve 2 g orcinol monohydrate (Sigma-Aldrich, St. Louis, MO) with 100 mL 2 N H2SO4 |
| ● |
Primuline reagent (dissolve 50 mg primuline (Kanto Chemical Co., Inc., Tokyo, Japan) in 10% acetone aq. solution (stock solution), and dilute the stock solution 100 times with 80% acetone aq. solution immediately before use |
|
Instruments
 |
| ● |
TLC thermal blotter (ATTO Corporation) |
| ● |
Densitometer (CS-9000, Shimadzu Corp., Kyoto, Japan) |
|
| Methods |
|
1. |
Cellular synthesis of oligosaccharides using saccharide primers |
| 1) |
Prepare 50 μM saccharide primers by diluting 20 mM saccharide primers in DMSO with serum-free and phenol red-free culture medium. |
Comment 0
|

|
| 2) |
Seed 2 × 106 cells into a 100-mm culture dish and culture in 5% CO2-95% air at 37ºC for 18 h. |
Comment 0
|

|
| 3) |
Replace the culture medium with 5 mL serum-free culture medium containing 50 μM saccharide primers. |
Comment 0
|

|
| 4) |
After 48 h, collect the culture medium. |
Comment 0
|

|
| 5) |
Collect the glycosylated products with a Sep-Pak C18 column. Absorb the glycosylated products to the Sep-Pak C18 column by eluting the collected culture medium twice. After washing the column with pure water, elute the glycosylated products with methanol. |
Comment 0
|

|
| 6) |
Evaporated the eluate containing the glycosylated products under reduced pressure. |
Comment 0
|

|
| 7) |
Separate the products into neutral and acidic products using amino column. Dissolve the product in 50 μL of methanol and 950 μL of chloroform. Absorb the products into the column by eluting the solution containing the products twice. After washing the column, collect the neutral product by eluting with 2 mL of ethanol, and collect the acidic products by eluting with 2 mL of 93:3:4 methanol-acetic acid-triethylamine. |
Comment 0
|

|
| 8) |
HPTLC
Separate the glycosylated products on an HPTLC plate by developing with 5:4:1 chloroform-methanol-0.2% CaCl2. After drying the HPTLC plate, spray with resorcinol reagent for acidic products or orcinol reagent for neutral products. Measure the tone of the stained band by a densitometer. |
Comment 0
|

|
| 9) |
TLC Blotting
Separate the glycosylated products on an HPTLC plate by developing with 5:4:1 chloroform-methanol-0.2% CaCl2. After drying the HPTLC plate, spray with primuline reagent, and mark the spots with a red pencil under UV light. Dip the HPTLC plate in a blotting solvent of 40:7:20 2-propanol-methanol-0.2% CaCl2 for 20 sec and placed on a glass fiber filter. Cover the plate with a PVDF membrane, a PTFE membrane and another glass fiber filter. Subject these layers to pressure at 180˚C for 30 sec using a TLC thermal blotter. Wash the PVDF membrane with pure water, and extract the products with methanol and 2:1 CHCl3-methanol. |
Comment 0
|

|
| 10) |
Isolation of products by HPLC
Dissolve the crude products in 70:28:2 CHCl3-methanol-H2O, and injected into an HPLC system equipped with an Iatrobead column. Separate neutral products by eluting with 70:28:2 CHCl3-methanol-H2O, and acidic products with 70:28:2 CHCl3-methanol-H2O and 60:35:5 CHCl3-methanol-H2O at 2 mL/min of flow rate. Collect the fractions at 30 sec intervals for 40 min. |
Comment 0
|
|
|
| Notes | In the steps 7), 8), and 10), the composition of the mixed solvent have to be modified as to the variety of the obtained glycosylated product when the separation efficiency is not sufficient.
Conversion of saccharide primers is dependent on the combination of saccharide primers and cell lines. In the standard cases, conversion was about 10%. In the case of one 100-mm culture dish, you can obtain the enough amounts of glycosylated products for the analyses by a HPTLC and a mass spectrometer. |
| Figure & Legends |
Figure & Legends 
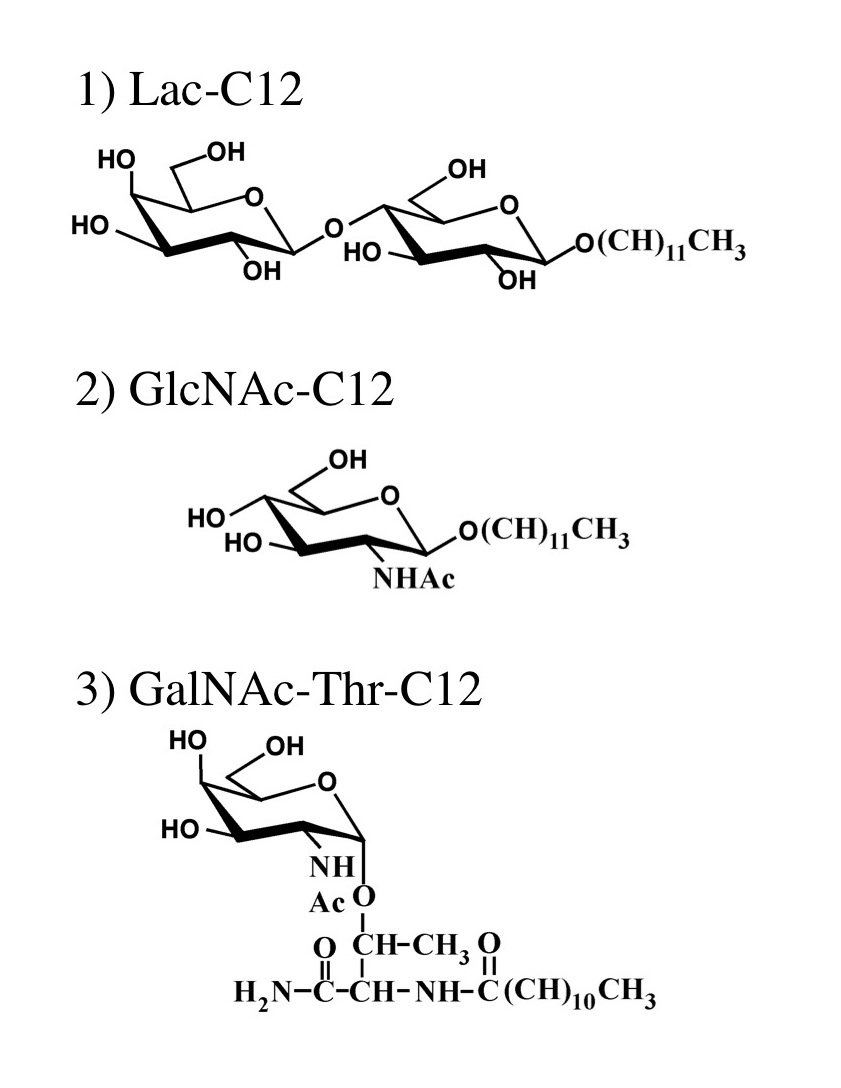
Fig. 1. Saccharide Primers suitable for the glycosylation in cells |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2015-01-19 11:42:15 |
- Miura, Y., and Yamagata, T. (1997) Glycosylation of lactosylceramide analogs in animal cells: amphipathic disaccharide primers for glycosphingolipid synthesis. Biochem. Biophys. Res. Commun. 241, 698–703 [PMID : 9434771]
- Nakajima, H., Miura, Y., and Yamagata, T. (1998) Glycosylation of amphipathic lactoside primers with consequent inhibition of endogenous glycosphingolipid synthesis. J. Biochem. 124, 148–156 [PMID : 9644257]
- Sato, T., Hatanaka, K., Hashimoto, H., and Yamagata, T. (2007) Syntheses of Oligosaccharides Using Cell Function. Trends In Glycoscience and Glycotechnology, 19, 1–17 [PMID : not available]
- Sato, T., Takashiba, M., Hayashi, R., Zhu, X., and Yamagata, T. (2008) Glycosylation of dodecyl 2-acetamido-2-deoxy-β-D-glucopyranoside and dodecyl β-D-galactopyranosyl-(1→4)-2-acetamido-2-deoxy-β-D-glucopyranoside as saccharide primers in cells. Carbohydr. Res. 343, 831–838 [PMID : 18262174]
- Kaneko, T., Okita, H., Nakajima, H., Iijima, K., Ogasawara, N., Miyagawa, Y., Katagiri, Y.U., Nakagawa, A., Kiyokawa, N., Sato, T., and Fujimoto, J. (2010) Newroblastoma cells can be classified according to glycosphingolipid expression profiles identified by liquid chromatography-tandem mass spectrometry. Int. J. Oncol. 37, 1279–1288 [PMID : 20878075]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Sato, Toshinori,
(2015). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Sato, Toshinori,
(2015).
Cellular synthesis of oligosaccharides using saccharide primers.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t146.
html source
Sato, Toshinori,
(2015).
<b>Cellular synthesis of oligosaccharides using saccharide primers</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t146" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t146</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|