Macrophage Galactose-type C-type lectin (MGL/CD301) is a type II transmembrane lectin. MGL has two homologous genes in mice, Mgl1 and Mgl2. MGL1 and MGL2 have unique carbohydrate specificity. Both MGL1 and MGL2 recoginize galactose (Gal) and N-acetylgalactosamine (GalNAc) as monosaccharides. However, the carbohydrate specificity to oligosaccharides is different between MGL1 and MGL2. MGL1 prefer to recognize LeX, whereas MGL2 prefer to recognize GalNAc residue (Tsuiji, et al. 2002). Recently, we generated monoclonal antibodies (mAbs) specific for MGL1 or MGL2. By the use of these mAbs, we examined expression of MGL1 and MGL2 on isolated cells of various tissue by flow cytometry and found MGL1 and MGL2 were expressed on some of macrophages and dendritic cells (Denda-Nagai et al 2010; Kumamoto et al 2009; Saba et al 2009). Here, we show MGL1 and MGL2 expression in cell suspension of peripheral lymph nodes. |
| Category | Sugar binding proteins |
| Protocol Name | Flow cytometric analysis of MGL1 and MGL2 expression by the use of specific monoclonal antibodies |
Authors
 |
Murakami, Ryuichi
Laboratory of Cancer Biology and Molecular Immunology, Graduate School of Pharmaceutical Sciences, The University of Tokyo
Denda-Nagai, Kaori
Laboratory of Cancer Biology and Molecular Immunology, Graduate School of Pharmaceutical Sciences, The University of Tokyo
Irimura, Tatsuro
*
Laboratory of Cancer Biology and Molecular Immunology, Graduate School of Pharmaceutical Sciences, The University of Tokyo
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Preparation of monoclonal antibodies specific for MGL1 or MGL2 is described elsewhere (Denda-Nagai, K et al. 2010; Kimura, T et al 1995). |
|
| Methods |
|
1. |
Cell preparation from peripheral lymph nodes |
| 1) |
Axillary, brachial, and inguinal lymph nodes (LNs) are obtained from naïve mice. |
Comment 0
|

|
| 2) |
LNs are minced and digested for 20 min at 37°C with 1 mg/mL collagenase from Clostridium histolyticum (Sigma-Aldrich, St. Louis, MO) in cRPMI (RPMI 1640 medium supplemented with 10% FCS and 10 mM HEPES). |
Comment 0
|

|
| 3) |
The digested LNs are re-suspended by pipetting. |
Comment 0
|

|
| 4) |
Phosphate buffered saline (PBS) is added to LN cell suspensions. |
Comment 0
|

|
| 5) |
LN cell suspensions are transferred to new tube through iron mesh and centrifuged. |
Comment 0
|

|
| 6) |
LN cells are treated with 10 mM EDTA for 5 min to inactivate collagenase and to disrupt T cell-DC complexes. |
Comment 0
|

|
| 7) |
PBS is added to LN cell suspensions. |
Comment 0
|

|
| 8) |
LN cell suspensions are transferred to a new tube through nylon mesh and centrifuged. |
Comment 0
|

|
| 9) |
LN cells are washed twice with PBS. |
Comment 0
|

|
| 10) |
LN cells are suspended in PBS containing 0.1% BSA and 0.1% sodium azide (FCM buffer). |
Comment 0
|
|
|
|
2. |
Staining of isolated LN cells and flow cytometric analysis |
| 1) |
Cells are incubated with anti-mouse CD16/CD32 (1/100 dilution of ammonium sulfate precipitated hybridoma culture supernatants of 2.4G2 hybridoma purchased from ATCC) to reduce nonspecific binding 5 min before the addition of the first antibodies. |
Comment 0
|

|
| 2) |
Cells are incubated with biotin-conjugated anti-MGL1 mAb LOM8.7 (bio-LOM8.7: 10 μg/mL), allophycocyanin-conjugated anti-MGL2 mAb URA-1 (APC-URA-1: 10 μg/mL), PE-Cy5-conjugated anti-CD3 (0.4 μg/mL) and PE-Cy5-conjugated anti-CD19 (eBioscience: 0.1 μg/mL) for 30 min on ice. |
Comment 0
|

|
| 3) |
Cells are washed with FCM buffer and centrifuged. |
Comment 0
|

|
| 4) |
Supernatants are removed and tubes are tapped until cells are separated. |
Comment 0
|

|
| 5) |
Cells are incubated with 0.2 μg/mL PE-Cy7-labeled streptavidin (SAV) (BioLegend, San Diego, CA) for 30 min on ice to visualize biotin-conjugated mAb LOM8.7. |
Comment 0
|

|
| 6) |
Cells are washed with FCM buffer and centrifuged. |
Comment 0
|

|
| 7) |
Supernatants are removed and tubes are tapped until cells are separated. |
Comment 0
|

|
| 8) |
Cells are re-suspended in FCM buffer containing 1 μg/mL 7-amino-actinomycin D (eBioscience, Inc., San Diego, CA) to exclude dead cells. |
Comment 0
|

|
| 9) |
Samples are analyzed on FACSAria cell sorter (BD, Franklin Lakes, NJ). |
Comment 0
|

|
| 10) |
Data are analyzed using FlowJo software (Tree Star, Ashland, OR). |
Comment 0
|
|
|
| Figure & Legends |
Figure & Legends 
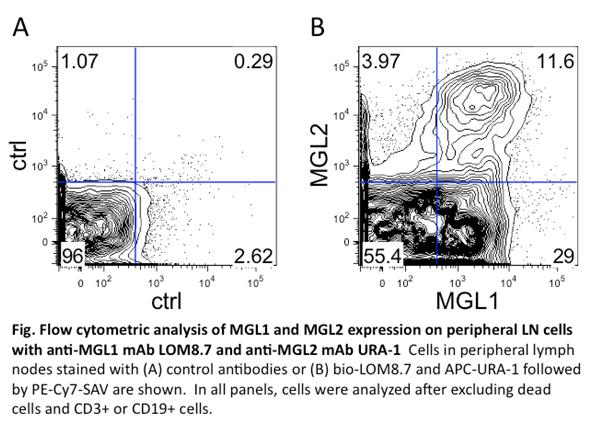
|
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-30 18:05:07 |
- Tsuiji, M., Fujimori, M., Ohashi, Y., Higashi, N., Onami, T.M., Hedrick, S.M., and Irimura, T. (2002) Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J Biol Chem. 277, 28892–901 [PMID : 12016228]
- Denda-Nagai, K., Aida, S., Saba, K., Suzuki, K., Moriyama, S., Oo-Puthinan, S., Tsuiji, M., Morikawa, A., Kumamoto, Y., Sugiura, D., Kudo, A., Akimoto, Y., Kawakami, H., Bovin, N.V., and Irimura, T. (2010) Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): Efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem. 285, 19193–204 [PMID : 20304916]
- Kumamoto, Y., Denda-Nagai, K., Aida, S., Higashi, N., and Irimura, T. (2009) MGL2 Dermal dendritic cells are sufficient to initiate contact hypersensitivity in vivo. PLoS One. 4, e5619 [PMID : 19440334]
- Saba, K., Denda-Nagai, K., and Irimura, T. (2009) A C-type lectin MGL1/CD301a plays an anti-inflammatory role in murine experimental colitis. Am J Pathol. 174, 144–52 [PMID : 19095961]
- Kimura, T., Imai, Y., and Irimura, T. (1995) Calcium-dependent conformation of a mouse macrophage calcium-type lectin. Carbohydrate binding activity is stabilized by an antibody specific for a calcium-dependent epitope. J Biol Chem. 270, 16056–62 [PMID : 7541793]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Murakami, Ryuichi,
Denda-Nagai, Kaori,
Irimura, Tatsuro,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.25,4,2024 .
How to Cite this Work in Website:
Murakami, Ryuichi,
Denda-Nagai, Kaori,
Irimura, Tatsuro,
(2014).
Flow cytometric analysis of MGL1 and MGL2 expression by the use of specific monoclonal antibodies.
Retrieved 25,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t141.
html source
Murakami, Ryuichi,
Denda-Nagai, Kaori,
Irimura, Tatsuro,
(2014).
<b>Flow cytometric analysis of MGL1 and MGL2 expression by the use of specific monoclonal antibodies</b>.
Retrieved 4 25,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t141" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t141</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|