Galectins are a group of lectins, of which structures are evolutionarily related and their carbohydrate-binding specificity is defined for β-galactoside, such as N-acetyllactosamine1). Therefore, galectins are generally purified on lactosyl-agarose or appropriate alternatives containing N-acetyllactosamine, e.g., asialofetuin-agarose. Conventionally, sugar-binding activity of galectins are evaluated by hemagglutination assay using glutaraldehyde-fixed, trypsin-sensitized rabbit erythrocyte, where galectin activity is substantially inhibited by the addition of β-galactosides, such as lactose and thiodigalactoside (<1 mM). Since the method is quite simple and easy to perform, it provides rather semi-quantitative data on sugar-binding activity and specificity, for which more solid and quantitative analytical approaches, such as frontal affinity chromatography2), are necessary. Although nowadays, most galectin proteins are discovered from genome information, it is still necessary to investigate natural galectins, especially from organisms, of which genome sequences are not available, e.g., Xenopus laevis. |
| Category | Sugar binding proteins |
| Protocol Name | Isolation and binding assay of galectins |
Authors
 |
Hirabayashi, Jun
Biotechnology Research Institute for Drug Discovery, Department of Life Science and Biotechnology, National Institute of Advanced Industrial Science and Technology (AIST)
|
| KeyWords |
|
Reagents
 |
| ● |
Affinity chromatography
- Fetuin (Gibco/Life Technologies, Carlsbad, CA)
- CN-activated Sepharose (GE Healthcare, Little Chalfont, UK)
- Lactose (Wako Pure Chemical Industries, Ltd., Osaka, Japan)
- MEPBS (4 mM β-mercaptoethanol, 2 mM EDTA, 20 mM Na-phosphate, pH 7.2)
|
| ● |
Hemagglutination assay
- Rabbit erythrocyte (freshly bled from the animal)
- Trypsin (Sigma-Aldrich, St. Louis, MO)
- Glutaraldehyde (Wako Pure Chemical Industries, Ltd.)
- 96-well microtiter plate, V shape (Greiner Bio-One GmbH, Frickenhausen, Germany)
|
|
Instruments
 |
|
| Methods |
|
1. |
Affinity chromatography: Preparation of asialofetuin-agarose3) |
| 1) |
Fetuin (500 mg)
↓dissolve in 50 mL of 0.1 M HCl in an appropriate tube with a screw cap.
↓adjust pH to 2 with 1 M HCl.
↓heat up to 80°C without a cap screwed.
↓incubate at 80°C for 1 h with a cap screwed.
↓cool down to <10°C without a cap screwed.
↓dialyze against 0.1 M NaHCO3 in a cold room (<10°C).
↓determine protein and sialic acid (see Comment ). |
Comment 1
|

|
| 2) |
Asialofetuin
↓dissolve in 0.1 M NaHCO3.
↓adjust pH to 9.0.
↓couple to CN-activated Sepharose (50 mL) to ensure immobilization according to the instruction supplied by the manufacturer (e.g., <1 h, at room temperature).
↓glass filtrate (see Comment). |
Comment 1
|

|
| 3) |
Asialofetuin-agarose resin
↓pack into an appropriate size of column for affinity chromatography (see Comment). |
Comment 1
|

|
| 4) |
Asialofetuin-agarose column
↓equilibrate with MEPBS in a cold room (<10°C). |
Comment 0
|
|
|
|
2. |
Affinity chromatography: Purification of galectin by asialofetuin-agarose chromatography |
| 1) |
Starting materials, such as tissue extracts containing galectin must be free from β-galactoside, such as lactose, which is used for extraction of galectin from tissues (see Comment*). A typical image of chromatogram is shown in Fig. 2.
Galectin-containing extracts (β-galactoside free)
↓apply to an asialofetuin-agarose column equilibrated with MEPBS.
↓extensively wash the column with MEPBS.
↓elute the bound galectin with 0.1 M lactose (see Comment**). |
Comment 1
|
|
|
|
3. |
Hemagglutination assay: Preparation of glutaraldehyde-fixed trypsinized rabbit erythrocytes |
| 1) |
The following method is based on the report by Nowak et al.4).
10 mL rabbit blood in 50 mL tube
↓extensively washed with cold PBS by centrifugation. |
Comment 1
|

|
| 2) |
Erythrocytes (ca. 5 mL)
↓suspend in 100 mL of 0.1% (w/v) trypsin solution in PBS.
↓37°C, 1 h with occasional shake.
↓extensively wash with cold PBS by centrifugation. |
Comment 0
|

|
| 3) |
Trypsinized erythrocytes
↓suspend in 25 mL of 1% glutaraldedehyde in PBS.
↓incubate at room temperature for 1 h with continuous gentle shake.
↓centrifuge. |
Comment 0
|

|
| 4) |
Erythrocytes
↓wash twice with 25 mL of 0.1 M glycine in PBS.
↓extensively wash with PBS.
↓store as 10% suspension in a cold room. |
Comment 0
|
|
|
|
4. |
Hemagglutination assay: Hemaggultination assay |
| 1) |
An image of a typical result of hemagglutination assay of galectins is shown in Fig. 4.
Microtiter plate
↓add 25 mL of a galectin-containing solution in the left well of a vertical line.
↓make a series of 2-fold dilution (2-1, 2-2, 2-3, …..) of the sample in MEPBS in the vertical line (see Comment*).
↓add 25 mL of either PBS or sugar solution dissolved in PBS to each well.
↓add 1% (w/v) bovine serum albumin to each well.
↓add 4% glutaraldehyde-fixed trypsinized rabbit erythrocytes prepared above.
↓allow to proceed hemagglutination reaction for 1 h at room temperature.
↓hemagglutination titer determined (see Comment**). |
Comment 1
|
|
|
| Notes | Instruments
Any conventional type will do for the above purposes, and there is no special requirement for makers.
Methods
Affinity chromatography
Affinity chromatography is a powerful method for rapid purification of lectins. In the case of galectins, affinity adsorbents carrying appropriate β-galactosides, such as lactose-agarose and asialofetuin agarose, have widely used. A general principle of affinity chromatography for galectin is schematically shown in Fig. 1. Preparation of asialofetuin-agarose is described first, and then a typical procedure for affinity chromatography is described.
Hemagglutination assay
Hemagglutination assay is a most conventional method to characterize lectins, which have multiple carbohydrate-binding sites, and thus, can cross-link various types of cells. For this purpose, erythrocytes from various species (rabbit, human, mouse, chick, etc.) have widely been used (Fig. 3). Though the method is merely semi-quantitative, it provides an extremely easy way to handle in a visible format on a microtiter plate. For this purpose, trypsin-sensitized rabbit erythrocytes are most often sued, and the sensitized cells can be stored for a long period (>1 month) by glutaraldehyde fixation. |
| Figure & Legends |
Figure & Legends 
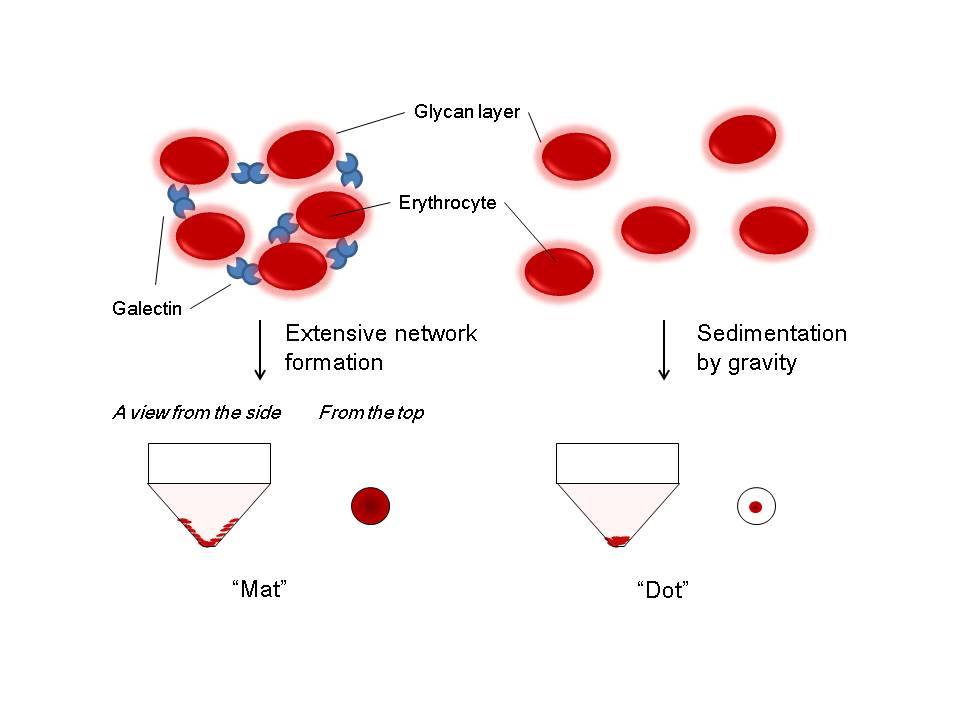
Fig. 1. Schematic representation of a principle of affinity chromatography for purification of galectin.
The procedure consists of three general processes, i.e., 1) adsorption of lectin protein on the column resin (in this case, asialofetuin-agarose), 2) extensive washing of the column to remove unbound materials, and 3) specific elution of the adsorbed galectin protein with a potent saccharide inhibitor (e.g., lactose).

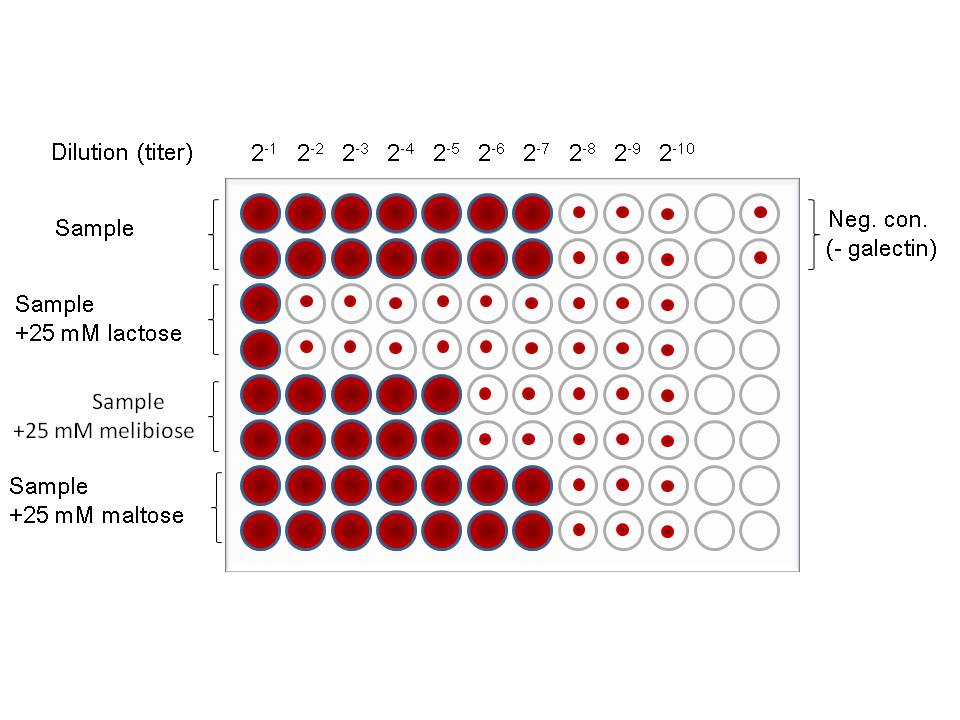
Fig. 2. A typical image of galectin purification by affinity chromatography on an asialofetuin-agarose column.
An approximate column volume of asialofetuin-agarose is 10–20 mL if one assumes to use asialofetuin agarose (10 mg/mL gel) for purification of 5–50 mg of galectin from a relatively large amount of tissue (100–500 g wet weight). After extensive washing of the column, the bound material (galectin) is specifically eluted with lactose (20–100 mM). Hemagglutinating activity of lactose-eluted fractions must be measured after removal of lactose by extensive dialysis.

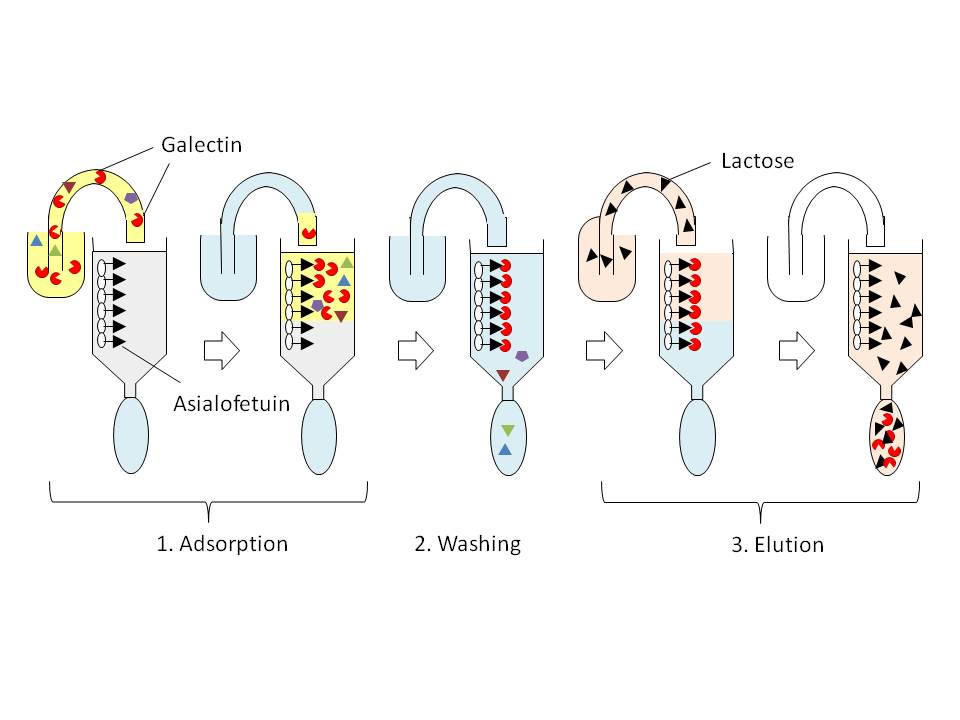
Fig. 3. Brief explanation of how hemagglutination assay can be performed.
In the presence of multivalent sugar-binding proteins (e.g., lectins and carbohydrate-specific antibodies), appropriately treated erythrocytes (e.g., glutaraldehyde-fixed, trypsin-sensitized rabbit erythrocytes) are agglutinated. They form apparent “mat” on the microtiter plate, while in the absence erythrocytes simply form sediment by gravity, and thus, they are observed as “dot” on the plate.

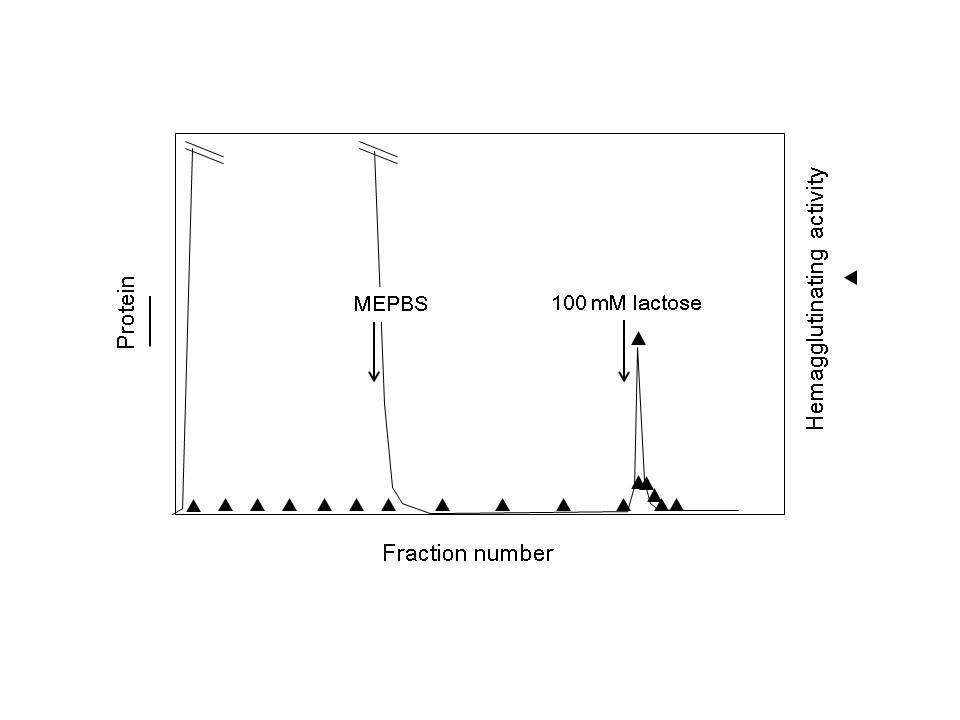
Fig. 4. An image of a typical result of hemagglutination assay of galectins.
The procedure consists of serial two-fold dilution of a sample containing galectin, addition of saccharide inhibitors and 1% (w/v) bovine serum albumin, and 4% erythrocytes. in this case, a representative saccharide inhibitor lactose (Galβ1-4Glc) and its structural isomers, melibiose (Galα1-6Glc) and maltose (Glcα1-4Glc) are used for comparison. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-10-23 11:31:20 |
- Barondes, S.H., Castronovo, V., Cooper, D.N., Cummings, R.D., Drickamer, K., Feizi, T., Gitt, M.A., Hirabayashi, J., Hughes, C., Kasai, K., et al. (1994) Galectins: a family of animal beta-galactoside-binding lectins. Cell. 76, 597–598 [PMID : 8124704]
- Hirabayashi, J., Hashidate, T., Arata, Y., Nishi, N., Nakamura, T., Hirashima, M., Urashima, T., Oka, T., Futai, M., Muller, W.E.G., Tagi, F., and Kasai, K. (2002) Oligosaccharide specificty of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 [PMID : 12223272]
- De Waard, A., Hickman, S., and Kornfeld, S. (1976) Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J. Biol. Chem. 251, 7581–7587 [PMID : 826531]
- Nowak, T.P., Haywood, P.L., and Barondes, S.H. (1971) Developmentally regulated lectin in embryonic chick muscle and a myogenic cell line. Biochem. Biophys. Res. Commun. 68, 650–657 [PMID : 1259723]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Hirabayashi, Jun,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Hirabayashi, Jun,
(2014).
Isolation and binding assay of galectins.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t123.
html source
Hirabayashi, Jun,
(2014).
<b>Isolation and binding assay of galectins</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t123" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t123</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|