Oligosaccharides linked to glycoproteins and glycopeptides play important roles in several biological events. Therefore, it is essential to synthesize glycoproteins and glycopeptides for use as probes to study their biological roles. In order to synthesize these molecules, suitably protected asparaginyl-sialyloligosaccharide is essential for solid phase peptide synthesis. This protocol will show an efficient preparation method of Fmoc- asparaginyl-sialyloligosaccharide. |
| Category | Large scale preparation of glycans, glycoproteins & glycolipids |
| Protocol Name | Large scale preparation of oligosaccharides from chicken eggs |
Authors
 |
Kajihara, Yasuhiro
Department of Chemistry, Osaka University
|
| KeyWords |
|
Reagents
 |
| ● |
Actinase-E (Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) |
| ● |
All chemical reagents (Merck Millipore, Billerica, MA or Sigma-Aldrich, St. Louis, MO) |
| ● |
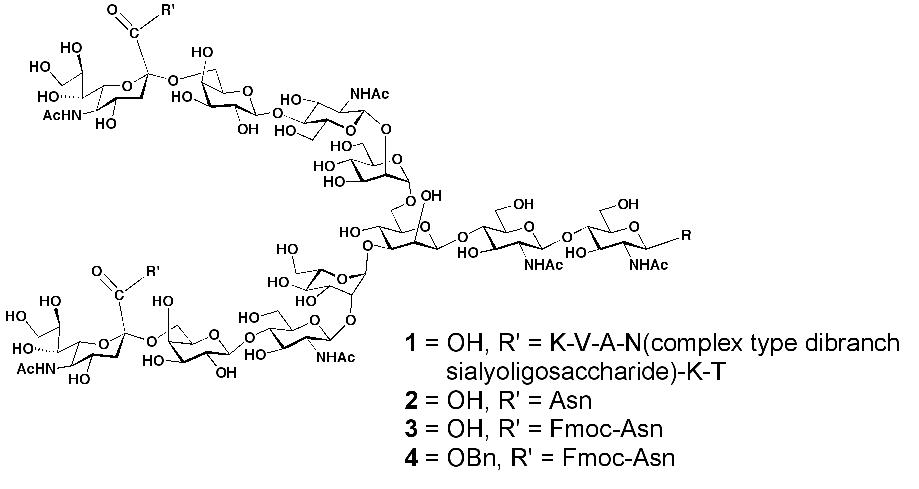
Scheme 1. Reagents:
a) Actinase-E, Tris-HCl buffer, NaN3, pH 7.5 (86%)
b) 9-fluorenylmethyl N-succinimidyl carbonate (Fmoc-OSu), NaHCO3, Acetone, H2O (68%)
c) Cs2CO3, H2O then BnBr, DMF (85%) |
|
| Methods |
|
1. |
|
| 1) |
Prepare egg yolks (12 pieces) after crash of eggshell. |
Comment 0
|

|
| 3) |
Add phenol solution (34.2 g phenol / 3.8 g water). |
Comment 0
|

|
| 6) |
Centrifuge (6000 rpm) for 30 min at 4°C. |
Comment 0
|

|
| 7) |
Filter the supernatant and then concentrate at 35°C. |
Comment 0
|

|
| 8) |
Prepare a solution of the residue in water (8 mL). |
Comment 0
|

|
| 9) |
Filter the solution by filter paper. |
Comment 0
|

|
| 10) |
Purify by gel permeation column (Sephadex G50, 0.1 M NaCl, 2.5 cm × 100 cm). |
Comment 0
|

|
| 11) |
Collect the fractions containing sialyloligosacchary peptide 1 and then concentrate. |
Comment 0
|

|
| 12) |
Desalt by Sephadex G50 column (water, id 2.5 cm × 100 cm). |
Comment 0
|

|
| 13) |
Collect the fractions containing sialyloligosacchary peptide 1 and then concentrate. |
Comment 0
|

|
| 14) |
Purify by DEAE-650M column (water, id 2.5 cm × 15 cm). |
Comment 0
|

|
| 15) |
Collect the fractions containing sialyloligosacchary peptide 1 and then concentrate. |
Comment 0
|

|
| 16) |
Isolate sialyloligosaccharyl peptide 1 (113 mg)1). |
Comment 0
|
|
|
|
2. |
Asparaginyl sialyloligosaccharide 2 |
| 1) |
Prepare a solution containing crude sialylglycopeptide 1 (809 mg) and NaN3 in a Tris-HCl buffer (50 mM, CaCl2 10 mM, pH 7.5, 32 mL). |
Comment 0
|

|
| 2) |
Add Actinase-E (263 mg) to a solution of sialylglycopeptide 1. |
Comment 0
|

|
| 3) |
Stir this solution for 60 h at 37°C (the pH was kept at 7.5). |
Comment 0
|

|
| 4) |
After 60 h, add Actinase-E (25 mg) again and then incubate for 55 h. |
Comment 0
|

|
| 6) |
Purify by gel permeation (Sephadex-G-25, 2.5 × 100 cm, H2O). |
Comment 0
|

|
| 7) |
Isolate sialyloligosacchary-asparagine 2 (301 mg). |
Comment 0
|
|
|
|
3. |
|
| 1) |
Prepare a solution containing asparagine linked sialyloligosaccharide 2 (80 mg, 0.034 mmol) and NaHCO3 (11.5 mg, 0.137 mmol) in H2O-acetone (2.7 mL–4.1 mL). |
Comment 0
|

|
| 2) |
Add a solution (acetone 4.1 mL) containing 9-fluorenylmethyl-N-succimidylcarbonate (34.7 mg, 0.103 mmol). |
Comment 0
|

|
| 3) |
Stir this mixture at room temperature for 2 h. |
Comment 0
|

|
| 4) |
Evaporate this mixture to remove acetone. |
Comment 0
|

|
| 5) |
Purify by reverse phase HPLC system (ODS-column, 1.6 × 14 cm, H2O to 20% MeOH). |
Comment 0
|

|
| 6) |
Isolate Fmoc-Asn-disialyloligosaccharide 3 (60 mg). |
Comment 0
|
|
|
|
4. |
|
| 1) |
Prepare a solution (cold H2O, 2 mL, 4°C) containing Fmoc-Asn-disialyloligosaccharide 3(20 mg). |
Comment 0
|

|
| 2) |
Desalt sodium salt from Fmoc-Asn-disialyloligosaccharide 3 by passing through a pasteur pipette column (0.5 cm × 5 cm) containing resin of Dowex-50W X8(H+) and then wash with water. |
Comment 0
|

|
| 4) |
Prepare a solution (water, 2 mL) containing Fmoc-Asn-disialyloligosaccharide 3. |
Comment 0
|

|
| 5) |
Add a solution of aqCs2CO3 (2.5 mg/1 mL) and adjust pH to 6. |
Comment 0
|

|
| 7) |
Prepare a solution (dry DMF, 1.3 mL) containing Fmoc-Asn-disialyloligosaccharide 3. |
Comment 0
|

|
| 8) |
Add BnBr (5.1 μL) to the above DMF solution and then stir for 45 h at room temperature. |
Comment 0
|

|
| 9) |
Add diethyl ether (10 mL) and then collect precipitate generated. |
Comment 0
|

|
| 10) |
Purify by ODS-column (1.6 cm × 14 cm, H2O to 40% MeOH). |
Comment 0
|

|
| 11) |
Isolate dibenzyl-sialyloligosaccharide 4 (18 mg). |
Comment 0
|
|
|
| Notes | Glycopeptide 1
This glycopeptide was prepared according to the reported conditions2).
Asparaginyl sialyloligosaccharide 2
Key points in this protocol are adjustment and keep of pH during enzymatic reaction. This reaction was monitored by TLC (1M NH4OAc:isopropanol = 1:1).
1H-NMR (400 MHz, 30°C in D2O, HOD = δ 4.81) δ 5.21 (s, 1H, Man4-H-1), 5.15 (d, 1H, J = 9.5 Hz, GlcNAc1-H-1), 4.03 (s, 1H, Man4’-H-1), 4.86 (s, 1H, Man3-H-1), 4.70 (m, 3H, GlcNAc2,5,5’-H-1), 4.53 (d, 2H, J = 8.0 Hz, Gal6,6’-H-1), 4.34 (bs, 1H, Man3-H-2), 4.28 (bd, 1H, Man4-H-2), 4.20 (bd, 1H, Man4’-H-2), 3.03 (dd, 1H, J = 4.4 Hz, 17.2 Hz, Asn-bCH2), 2.95 (dd, 1H, J = 7.0 Hz, 17.2 Hz, Asn-bCH2), 2.76 (bdd, 2H, J = 4.6 Hz, 12.4 Hz, NeuAc7,7’-H-3eq), 2.16 (s, 3H, Ac), 2.15 (s, 6H, Ac×2), 2.28 (s, 6H, Ac×2), 2.10 (s, 3H, Ac), 1.80 (dd, 2H, J = 12.4 Hz, 12.4 Hz, NeuAc7,7’-H-3ax)
Synthesis of 3
1H-NMR (400 MHz, 30°C in D2O, HOD = δ 4.81) δ 8.01 (d, 2H, J =7.5 Hz, Fmoc), 7.80(d, 2H, J = 7.5 Hz, Fmoc ), 7.60 (dd, 2H, J = 7.5 Hz, Fmoc), 7.53 (dd, 2H, J = 7.5 Hz, Fmoc), 5.22 (s, 1H, Man4-H-1), 5.09 (d, 1H, J = 9.4 Hz, GlcNAc1-H-1), 5.03 (s, 1H, Man4’-H-1), 4.86 (s, 1H, Man3-H-1), 4.69 (m, GlcNAc2,5,5’-H-1), 4.53 (d, 2H, J = 7.8 Hz, Gal6, 6’-H-1), 4.44 (1H, Fmoc), 4.34 (bd, 1H, Man3-H-2), 4.29 (bd, 1H, Man4-H-2), 4.20 (bd, 1H, Man4’-H-2), 2.83-2.72 (m, 3H, Asn-bCH2, NeuAc7,7’-H-3eq), 2.61 (bdd, 1H, Asn-bCH2), 2.15 (s, 9H, Ac×3), 2.12 (s, 6H, Ac×2), 1.98 (s, 3H, Ac), 1.80 (dd, 2H, J = 12.1 Hz, 12.1 Hz, NeuAc7,7’-H-3ax); HRMS Calcd for C103H154N8NaO66[M+Na+] 2581.8838, found 2581.8821
Synthesis of 4
1H-NMR (400 MHz, 30°C in D2O, HOD = δ 4.81) δ 8.00 (d, 2H, Fmoc), 7.80 (d, 2H, Fmoc), 7.65-7.50 (m, 12H, Ph, Fmoc), 5.46 (d, 2H, J = 11.6 Hz, PhCH2), 5.40 (d, 2H, J = 11.6 Hz, PhCH2), 5.21 (s, 1H, Man4-H-1), 5.08 (d, 1H, J = 9.3 Hz, GlcNAc1-H-1), 5.02 (s, 1H, Man4’-H-1), 4.86 (s, 1H, Man3-H-1), 4.67 (m, 3H, GlcNAc2,5,5’-H-1), 4.41 (bd, 3H, Gal6, 6’- H-1, Fmoc), 4.33 (bd, 1H, Man3- H-2), 4.27 (bd, 1H, Man4’-H-2), 4.20 (d, 1H, Man4- H-2), 2.79 (bd, 3H, Asn-bCH2, NeuAc7, 7’- H3eq), 2.61 (bdd, 1H, Asn-bCH2), 2.15 (s, 3H, Ac), 2.12 (s, 6H, Ac×2), 2.10 (s, 6H, Ac×2), 1.98 (s, 3H, Ac), 1.93 (2H, dd, J = 12.2, 12.2 Hz, NeuAc7,7’-H-3ax);HRMS Calcd for C117H165N8Na2O66[M+Na+] 2783.9597, found 2783.9501 |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2016-10-07 10:02:52 |
- Seko, A., Kotetsu, M., Nishizono, M., Enoki, Y., Ibrahim, H.R., Juneja, L.R., Kim, M. and Yamamoto, T. (1997) Occurence of a sialylglycopeptide and free sialylglycans in hen's egg york. Biochim Biophys Acta, 1335, 23–32 [PMID : 9133639]
- Yamamoto, N., Ohmori, Y., Sakakibara, T., Sasaki, K., Juneja, L.R., Kajihara, Y. (2003) Solid-phase synthesis of sialylglycopeptides through selective esterification of the sialic acid residues of an Asn-linked complex-type sialyloligosaccharide. Angew. Chem. Int. Ed Engl. 42, 2537–2540 [PMID : 12800181]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Kajihara, Yasuhiro,
(2016). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Kajihara, Yasuhiro,
(2016).
Large scale preparation of oligosaccharides from chicken eggs.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t115.
html source
Kajihara, Yasuhiro,
(2016).
<b>Large scale preparation of oligosaccharides from chicken eggs</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t115" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t115</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|