Hematogenous metastasis of cancer is a highly complex process consisting of multiple steps. It starts with the intravasation of cancer cells into the bloodstream from the primary tumor lesion. The cancer cells then travel in the blood stream, where they interact with various blood cells such as leukocytes. Finally, they adhere to endothelial cells somewhere in the peripheral vessel walls. After extravasation, they enter the connective tissue and form a new metastatic lesion.
In the extravasation step at the peripheral vessel walls, cancer cells having a higher adhesive affinity for endothelium have more opportunity to develop metastasis, escaping the killing activity of the monocytes and NK cells. The cancer-associated glycans, sialyl Lewis A and sialyl Lewis X, serve as ligands for cell adhesion molecules of the selectin family, such as E-selectin, which is expressed on vascular endothelial cells. These glycans are involved in the adhesion of cancer cells to vascular beds, and contribute to hematogenous metastasis of cancer. The degree of expression of these glycans at the surface of cancer cells is well correlated with the frequency of hematogenous metastasis and prognostic outcome of patients with cancers. |
| Category | Matrices & cellular trafficking |
| Protocol Name | Selectin-mediated cell adhesion assay of tumor cells |
Authors
 |
Miyazaki, Keiko
Division of Molecular Pathology, Aichi Cancer Center
Kimura, Naoko
Division of Molecular Pathology, Aichi Cancer Center
Kannagi, Reiji
*
Division of Molecular Pathology* / Research Complex for the Medical Frontiers**, Aichi Cancer Center* / Aichi Medical University**
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Preparation of cells expressing selectins
- Appropriate cultured endothelial cells (for instance, human umbilical vein endothelial cells, HUVECs) are grown in the monolayer at the bottom of 24-well plates in the presence of 1.0 ng IL1-β for 4 h. HUVECs at 2–6 passages after isolation will show the best response to IL-1β. Endothelial cells cultured without IL1-β serve as negative control cells.
- Cultured cells transfected with E-selectin cDNA (for instance, E-sel/CHO cells) can be used instead of endothelial cells. In such case, mock transfectant cells can serve as control cells.
|
| ● |
Preparation and labeling of cancer cells
- Cancer cells are harvested, resuspended in RPMI without FCS at 5×106 cells/mL, and labeled with BCECF-AM (2'7'-bis-(carboxyethyl)-5-(and-6)- carboxyfluorescein acetoxymethyl ester) at a final concentration of 5 nM for 30 min at 37°C in the dark.
- After labeling, the cells are washed twice with RPMI with 10% FCS, and are resuspended in the same medium.
|
|
Instruments
 |
| ● |
A rotary plate shaker: we use a small-size rotary shaker NR-3 (TAITEC Co., Ltd., Koshigaya, Japan). |
| ● |
A 24-well plate reader: we use an Arvo 1420 multi-label counter (Wallac, Gaithersburg, MD). |
|
| Methods |
|
1. |
Selectin-mediated cell adhesion assay of tumor cells |
| 1) |
Remove the medium from the 24 well plate, and add the labeled cancer cells at 0.5–1.0×106 cells/0.5 mL/well. |
Comment 0
|

|
| 2) |
Place the 24-well plate on a rotating platform (Taitec NR-3) for incubation under shear (90–180 rpm) for 20 min at room temperature in the dark. |
Comment 0
|

|
| 3) |
Remove the medium containing non-adherent cells, and wash the wells gently twice with PBS containing Ca2+ and Mg2+. |
Comment 0
|

|
| 4) |
Add 0.4 mL of 1% NP-40 in RPMI with 10% FCS to each well to lyse the adherent cells. |
Comment 0
|

|
| 5) |
Measure the fluorescence intensity of each well using a plate reader using a filter set for BCECF-AM (the same filter set as used for FITC). |
Comment 0
|

|
| 6) |
Calculate the number of adherent cells in each well using a reference curve made from fluorescence intensity of known number of labeled cancer cells. |
Comment 0
|
|
|
| Notes | This cell adhesion protocol is called a non-static monolayer cell adhesion assay, because of continuous mechanical rotation of the plate applied throughout the incubation period. This is based on that carbohydrate-mediated cell adhesion is relatively resistant to mechanic shear stress, while cell adhesion mediated by proteins such as integrins generally cannot withstand mechanical stress.
During the washing procedures (step 3), the plate can be checked under a microscope, to confirm that the washings were done gently enough, making sure that adherent cells do not detach from endothelial cells at the bottom of the wells. After the washing procedure, the plate can be checked again under a microscope, to make sure that any non-adherent cells do not remain floating in the medium.
Inhibitory antibodies such as anti-E-selectin (for treatment of endothelial cells), or antibodies directed to carbohydrate ligands (for treatment of cancer cells) can be added to the wells (Fig. 1) to ensure which molecules are involved in the adhesion, depending on the purpose of the experiments. |
| Figure & Legends |
Figure & Legends 
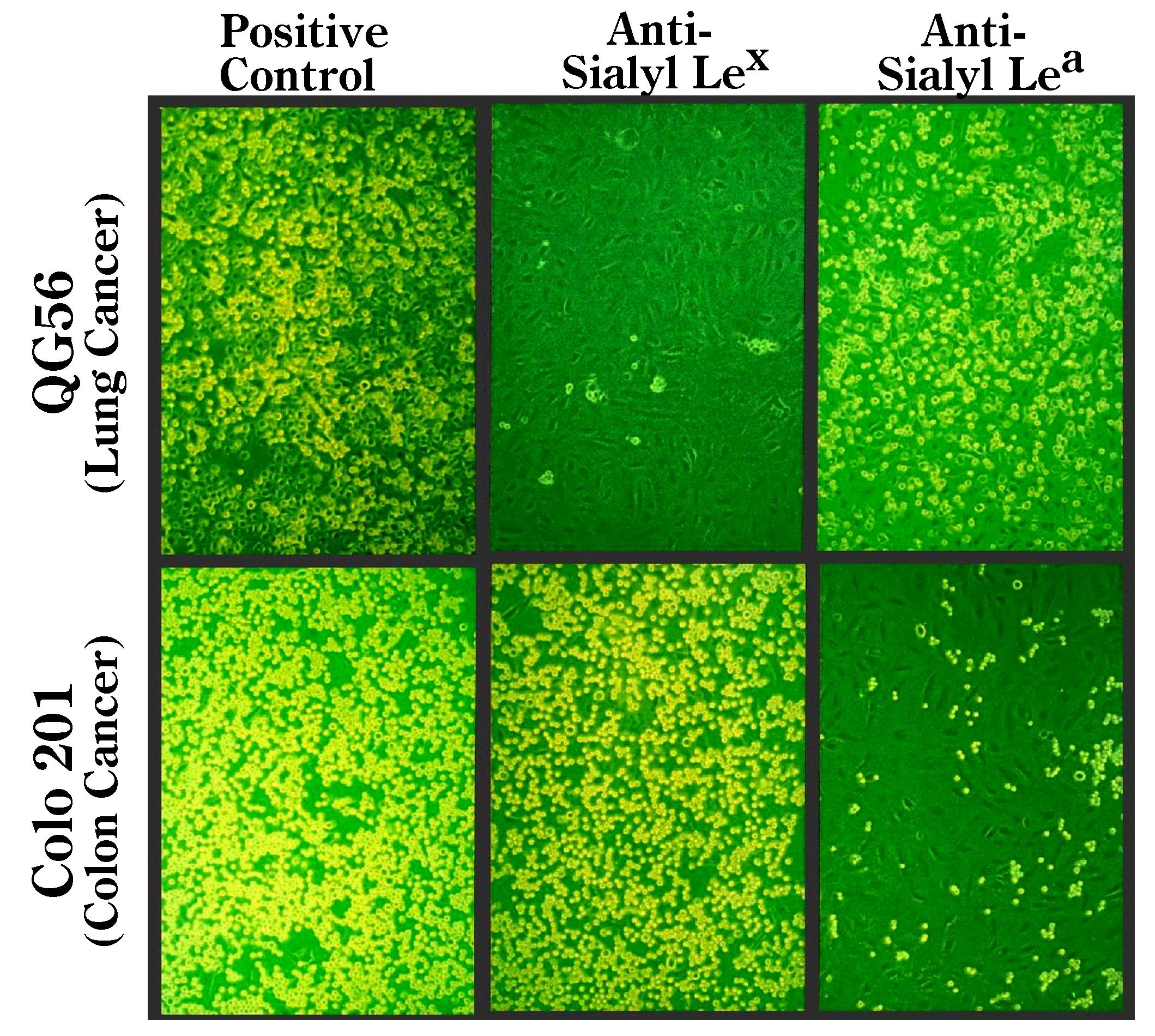
Fig. 1. Non-static monolayer cell adhesion assay using human cancer cells and typical inhibition patterns of the adhesion to recombinant IL-1β-activated HUVECs by treatment with anti-sialyl Lewis X, and anti-sialyl Lewis A antibodies.
Cancer cells were treated with the respective antibodies (20 μg/mL) for 30 min prior to the adhesion experiment. Results for QG-56 (lung cancer), and Colo201 (colon cancer) cells are shown. Note that adhesion of QG-56 is inhibited by anti-sialyl Lewis X antibody, while that of Colo201 cells is inhibited by anti-sialyl Lewis A antibody.
This figure was originally published in Cancer Res. Takada A, Kannagi R. et al. “Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium” 1993, 53(2):354–61. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-11-12 09:48:40 |
- Kannagi, R., Sakuma, K., Miyazaki, K., Lim, K.T., Yusa, A., Yin, J., and Izawa, M. (2010) Altered expression of glycan genes in cancers induced by epigenetic silencing and tumor hypoxia: Clues in the ongoing search for new tumor markers. Cancer Sci. 101, 586–593 [PMID : 20085584]
- Miyazaki, K., Ohmori, K., Izawa, M., Koike, T., Kumamoto, K., Furukawa, K., Ando, T., Kiso, M., Yamaji, T., Hashimoto, Y., Suzuki, A., Yoshida, A., Takeuchi, M., and Kannagi, R. (2004) Loss of disialyl Lewisa, the ligand for lymphocyte inhibitory receptor Siglec-7, associated with increased sialyl Lewisa expression on human colon cancers. Cancer Res. 64, 4498–4505 [PMID : 15231659]
- Kannagi, R., Izawa, M., Koike, T., Miyazaki, K., and Kimura, N. (2004) Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 95, 377–384 [PMID : 15132763]
- Takada, A., Ohmori, K., Yoneda, T., Tsuyuoka, K., Hasegawa, A., Kiso, M., and Kannagi, R. (1993) Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 53, 354–361 [PMID : 07678075]
- Takada, A., Ohmori, K., Takahashi, N., Tsuyuoka, K., Yago, K., Zenita, K., Hasegawa, A., and Kannagi, R. (1991) Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. Biochem. Biophys. Res. Commun. 179, 713–719 [PMID : 01716885]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Miyazaki, Keiko,
Kimura, Naoko,
Kannagi, Reiji,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.25,4,2024 .
How to Cite this Work in Website:
Miyazaki, Keiko,
Kimura, Naoko,
Kannagi, Reiji,
(2014).
Selectin-mediated cell adhesion assay of tumor cells.
Retrieved 25,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t112.
html source
Miyazaki, Keiko,
Kimura, Naoko,
Kannagi, Reiji,
(2014).
<b>Selectin-mediated cell adhesion assay of tumor cells</b>.
Retrieved 4 25,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t112" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t112</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|